污水处理厂、造纸厂、炼油厂(Iranpour et al., 2005; Karnofski et al., 1975; Smet et al., 1998; Bosch et al., 2009; Sipma et al., 2005) 及一些化工厂在生产过程中会释放大量挥发性有机硫化物(Volatile Organic Sulfur Compounds, VOSCs), 如甲硫醇(Methanethiol, MT)、乙硫醇(Ethanethiol, ET)、甲硫醚(Dimethyl Sulfide, DMS)和二甲基二硫醚(Dimethyl Disulfide, DMDS)等.上述VOSCs中, MT嗅阈值最低, 仅为0.0012~36 μg·m-3(Chan, 2006), 因而被列为硫系脱臭的重点(林琳, 2000) .
与其他VOSCs相比, MT具有很强的亲核性, 位阻大(Bosch et al., 2009), 且具有高毒性, 因而处理难度较大.常用的MT处理方法主要有:催化氧化法(Hulea et al., 2014) 、化学吸收法(Couvert et al., 2006) 、活性炭吸附(Bashkova et al., 2002)和生物法(Cáceres et al., 2012; Lebrero et al., 2014)等.其中, 生物法因具备反应条件温和、能耗少、运行费用低、二次污染小等优点而备受关注.与纯菌降解相比, 由特定污染物富集所得的混合菌群因具有高耐受和互营养的生物特性, 可通过共代谢、直接氧化等多种途径更有效地降解污染物(Zhang et al., 1992;杨丽卿, 2013) .基于此, 本研究从处理DMS和丙硫醇(PT)混合废气的生物滴滤塔中富集出一组能以MT为唯一碳源和硫源的混合菌群, 并对其降解特性进行考察, 分析MT的降解途径, 以期为进一步利用该混合菌群处理MT废气奠定基础.
2 材料与方法(Materials and methods) 2.1 培养基无机盐培养基:Na2HPO4·12H2O 4.5 g·L-1, KH2PO4 1.0 g·L-1, NH4Cl 1.5 g·L-1, MgCl2 0.2 g·L-1, CaCl2 0.023 g·L-1, 微量元素母液1 mL·L-1.所述微量元素母液浓度(g·L-1)组成:FeCl2 1.0, H3BO3 0.014, MnCl2 0.10, ZnCl2 0.10, Na2MoO4·2H2O 0.02, CoCl2·6H2O 0.02.
2.2 混合菌群的富集筛选从实验室处理DMS和PT混合废气的生物滴滤塔中取出滤料, 滤料上附着的生物膜取下后加入到325 mL的摇瓶中, 该摇瓶装有50 mL已灭菌的无机盐培养基(灭菌温度110 ℃, 灭菌时间40 min), 之后加盖密封橡胶塞, 采用已灭菌的气体注射器加入适量MT, 使其浓度为20 mg·L-1, 将摇瓶置于30 ℃, 160 r·min-1摇床培养.待MT降解完以后, 再转接到新鲜的含MT的无机盐培养液中, 接种量为1 mL.如此转接数代, 待MT降解时间和速度稳定以后, 即初步筛选到MT降解菌群.
进一步对获得的混合菌群进行传代培养, 考察其生长和降解性能的稳定性.实验操作与驯化富集过程一致.每转接1次, 认为是传代1次, 每次传代时间间隔为3 d.测定每次传代降解后MT和菌体的浓度.
2.3 菌群降解性能优化 2.3.1 培养条件的优化实验考察了温度(25、30、35、40、45 ℃)、初始pH (5.0、6.0、7.0、8.0、9.0、10.0) 对混合菌群降解MT性能的影响.在含20 mg·L-1 MT的无机盐培养基中加入1 mL初始OD600为0.01的混合菌液, 同时以不加混合菌液的体系为空白对照, 密封后置于相应条件下恒温振荡培养3 d.实验过程中定时取样, 测定MT及DMDS浓度和菌体浓度.所有实验组均设置3个平行样.
2.3.2 培养基的优化在最佳培养条件下, 考察外加碳源对混合菌群降解MT的影响.在含20 mg·L-1 MT的无机盐培养基中分别添加0.1 g·L-1的酵母膏(Yeast Extract, YE)、葡萄糖和柠檬酸, 同时加入1 mL初始OD600为0.01的混合菌液, 同时以不加混合菌液的体系为空白对照, 密封后置于相应条件下恒温振荡培养3 d.实验过程中定时取样, 测定MT及DMDS浓度和菌体浓度.上述实验均设置3个平行样.
2.4 菌群群落结构分析在50 mL已灭菌的无机盐培养中接入1 mL传代培养至第10代的混合菌液, 同时加入MT, 使其浓度为20 mg·L-1, 待MT及其氧化产物DMDS降解完全后, 将混合菌液交由生工生物工程(上海)股份有限公司进行高通量测序, 对其菌群群落结构进行分析.高通量测序是基于目前主流的MiSeq平台对16S rRNA基因高突变区序列进行测序, 测序区域选择V3~V4区, 测序片段为465 bp, 测序引物为341F (CCCTACACGA CGCTCTTCCGATCTG CCTACGG GNGGCWGCAG)\805R (GACTGGA GTTCCTTGGCA CCCGAGAATTCCAGACTACHVGGGTATCTAATCC), 使用Cutadapt、PEAR、Prinseq软件对测序数据进行处理, 获得干净数据.在Usearch 软件平台中运用Usearch的算法获得Hits序列, 然后使用Uchime鉴定嵌合体, 随后与数据库代表序列进行Alpha 多样分析, 使用97%相似度的OUT, 利用Mothur 做Rarefaction 分析, 利用R语言工具制作Rarefraction和Rank-abundant曲线.最终使用RDP Classifier和Blast 软件得到菌群物种丰度统计信息.
2.5 分析方法 2.5.1 MT和DMDS的测定采用岛津GC-2014气相色谱进行分析, 色谱柱型号为RTX-1(30 m×0.32 mm×25 μm);汽化室、柱温和FPD检测器温度分别为230、40、和230 ℃;载气为氮气, 总流量为16.5 mL·min-1, 柱流量为0.79 mL·min-1;氢气压和空气压分别为80 kPa和40 kPa;进样量为800 μL.
2.5.2 CO2的测定采用Agilent 6890气相色谱仪进行分析, HP-Plot-Q 色谱柱(30 m×0.32 mm×20 μm)及TCD检测器进行检测, 进样口及检测器温度均为100 ℃, 柱温恒定在40 ℃, 氦气作为载气, 流速为5 mL·min-1.
2.5.3 菌体浓度采用Hitachi U-2910型紫外/可见分光光度计, 在600 nm 波长下测定菌体的吸光度(OD600), 再根据光密度与细胞干重间的线性关系, 对其进行计算转化.
2.5.4 中间产物因降解过程中中间产物同时存在于气液两相, 需同时测定气相和液相中的中间产物.
气相中的产物:采用Agilent 7890A气相色谱-质谱仪进行分析, 色谱柱为HP-INNOWAX (30 mm×0.320 mm×0.50 μm);升温程序为35 ℃保留5 min, 5 ℃·min-1升高到40 ℃并保留5 min, 10 ℃·min-1升高到80 ℃并保留5 min, 20 ℃·min-1升高到160 ℃并保留5 min;质谱条件为EI源, 电离能量70 eV, 离子源230 ℃, 辅助温度280 ℃, 倍增电压0 V, 扫描质量数29~250 amu.
液相中的产物:采用Agilent 1200高效液相色谱和DIONEX2000离子色谱仪进行分析.C18柱(4.6 nm×250 nm), 柱温30 ℃, 检测波长UV为254 nm, 进样量20 μL, 流动相甲醇:水= 95:5, 流速1.0 mL·min-1;Ionpac AS19-HC型分离柱和Ionpac AS19-HC型保护柱, 淋洗液为KOH (浓度梯度为10~40 mmol·L-1), 电导检测器30 ℃, 流速1.00 mL·min-1, 进样量25 μL.
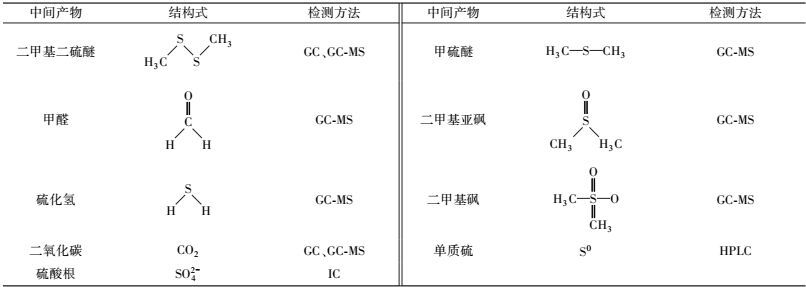
3 结果与讨论(Results and discussion) 3.1 混合菌群的富集筛选和传代实验经过多次驯化, 富集得到一组能以MT为唯一碳源和硫源的混合菌群.如图 1所示, 该混合菌群在70 h内能将20 mg·L-1 MT降解完全.菌体浓度从0.64 mg·L-1增至2.87 mg·L-1, 说明该混合菌群能以MT为唯一碳源和硫源生长.在MT的降解过程中, 发现有DMDS的积累, 且在50.5 h时达到最大值4.33 mg·L-1, 但随后DMDS浓度下降.DMDS的形成部分是MT自氧化作用的结果(Sipma et al., 2004; Bosch et al., 2009) .但因DMDS同样也是一种具有极低嗅阈值(仅为0.1 μg·m3)的恶臭物质(Rosenfeld et al., 2001), 故考察MT降解性能时, 需同时考察其氧化产物DMDS的降解情况.在降解实验完成后, 测得CO2最终浓度为1.91 mg·L-1, 可知MT的碳矿化率为58.6%, 表明该混合菌群对MT具有较高的矿化能力.
 |
| 图 1 混合菌群MT降解曲线与生长曲线 Fig. 1 Cell growth and MT degradation curve |
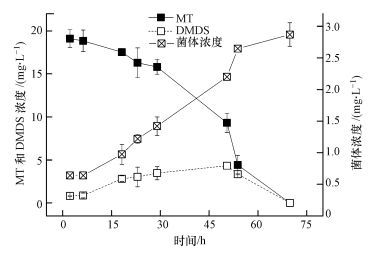
由图 2可以看出, 甲硫醇降解混合菌群经过连续20代的传代培养, MT的去除率均能保持在97%以上, 且无DMDS的积累, 这表明该混合菌群对MT降解性能稳定.
 |
| 图 2 MT去除率与传代次数的关系 Fig. 2 Effects of different generation on MT degradation |
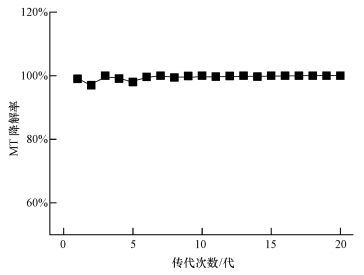
pH和温度等培养条件对微生物降解影响较大, 因此, 考察了其对混合菌群生长和降解性能的影响.由图 3可知, 在pH=7.0、温度为30 ℃的条件下, 降解性能最好, 菌体生长情况最佳.经过3 d时间的降解, MT及其氧化产物均被混合菌群降解, 菌体浓度增加至2.23 mg·L-1.在过酸或者过碱的环境下, 降解性能均不如中性条件.这可能因为不适的pH反应体系可能会改变微生物原生质膜的电荷, 以及影响降解酶的正常分泌(李政等, 2011) .在25~45 ℃温度范围内, 随着温度的升高, MT去除率也随之增加;但温度高于30 ℃后, 去除率及菌体浓度均下降.该实验现象可能是因为低温或者高温体系可能会使降解酶失活甚至变性, 进而影响MT的处理效果(刘洪霞等, 2013) .
 |
| 图 3 pH (a)和温度(b)对菌群生长和MT降解的影响 Fig. 3 Effects of pH (a) and temperature (b) on cell growth and MT degradation |
向反应体系中添加外加碳源, 如葡萄糖、YE等可使菌体迅速增殖, 从而达到增加降解效率的目的(王艳青等, 2009; 肖巧巧等, 2014) .同时, 外加碳源在降解过程中还具有提供电子供体、诱导产生降解酶等作用(张锡辉等, 2000) .本研究在无机盐培养基中分别添加YE、柠檬酸和葡萄糖这3种碳源, 研究不同碳源对混合菌群降解性能的影响.由图 4a可知, 添加外加碳源YE, 混合菌群对MT的降解性能与图 1不加外加碳源相比有显著的提高, 降解20 mg·L-1 MT所需时间缩短10 h.这可能是由于YE中同时富含蛋白质、氨基酸及微量元素等多种营养成分, 使混合菌群中功能菌迅速增殖, 从而加速MT的降解.但添加柠檬酸和葡萄糖, 虽然菌体浓度增加明显, 分别增加到10.32 mg·L-1和10.33 mg·L-1, 但其对MT的降解性能却无明显增加, 甚至因菌群优先利用易降解碳源(朱婷婷等, 2012), 出现MT降解速率下降的现象.
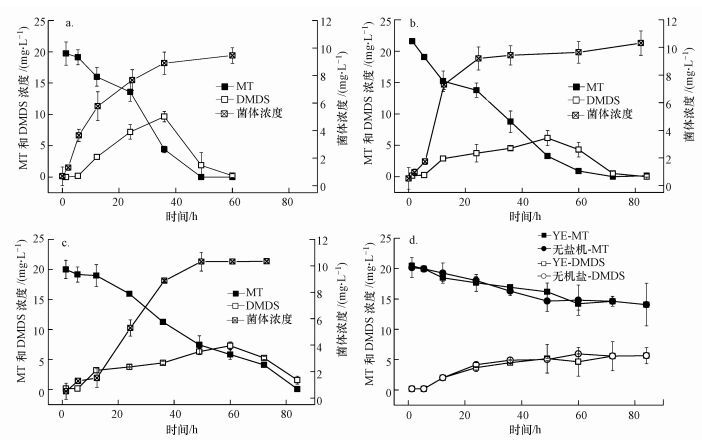 |
| 图 4 外加碳源对菌群降解MT的影响 (a.YE+菌, b.柠檬酸+菌, c.葡萄糖+菌, d.无菌) Fig. 4 Effects of exotic carbon on the MT degradation |
有氧气存在的情况下, MT可自氧化形成DMDS (Bosch et al., 2009; van Leerdam et al., 2008) .从图 4d可知, 添加外加碳源YE对MT自氧化速率无明显影响.但对比图 4a和图 4d可知, 在有混合菌群添加的情况下, MT向DMDS氧化加速, DMDS在36 h时积累量为9.75 mg·L-1.同时间点, 因自氧化产生的DMDS浓度仅为4.88 mg·L-1.这说明混合菌群中的微生物可通过生物氧化作用, 加速MT向DMDS的氧化进程, 从而加速MT的去除.
3.4 菌群群落结构分析为分析混合菌群群落结构, 对其进行微生物高通量测序, 在属水平上的优势菌属组成鉴定结果如图 5所示.可以看出, Pseudomonas sp.、Thiobacillus sp.、Acinetobacter sp.所占比例较高, 分别为33.78%、21.91%、17.01%, 另外还包含Hyphomicrobium sp.(2.8%)等.Thiobacillus属细菌常具有良好的H2S降解能力, 如T. thioparus TK-m (Tanji et al., 1989) 、T. thioparus DW44(Park et al., 1993) 、Thiobacillus HA43(Cho et al., 1991)等, 且T. thioparus TK-m、T. thioparus DW44还能同时降解MT和DMS.Pseudomonas属细菌、Acinetobacter属细菌和Hyphomicrobium属细菌在生物处理VOSCs类污染物中常有报道:Ito等(2014) 发现, P. fluorescens strain 76可降解MT和DMDS, P. acidovorans DMR-11(Zhang et al., 1991)和P. putida strain DS1(Shu et al., 2009) 则被发现能降解DMS;Horinouchi等(1997) 发现, Acinetobacter sp.20B能氧化DMS;Hyphomicrobium S (De et al., 1981) 、Hyphomicrobium EG (Suylen et al., 1986) 、Hyphomicrobium I55(Zhang et al., 1991) 对DMSO均有降解能力, 而Hyphomicrobium VS (Pol et al., 1994) 可用于降解DMS.因混合菌群中同时存在多种具有VOSCs类污染物降解能力的功能菌, 使得混合菌群具有良好及稳定的MT降解能力.
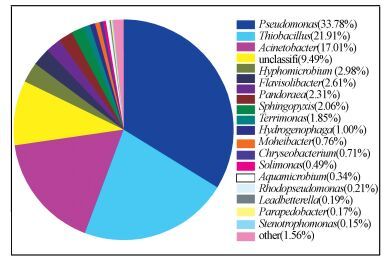 |
| 图 5 菌群群落结构(属水平) Fig. 5 The bacterial community structure at genus level |
在含20 mg·L-1 MT的无机盐培养基中添加1 mL初始OD600为0.01的混合菌液, 在30 ℃、初始pH为7.0的情况下置于摇床培养.在培养过程中, 采用GC-MS、IC、HPLC等分析方法对菌群降解中间产物进行定性分析, 推测可能的MT生物降解途径.实验中检测到的中间产物见表 1.
| 表 1 中间产物及分析方法 Table 1 metabolic intermediates and analytic methods |
根据检测所得的中间产物, 并且结合相关文献报道, 推断混合菌群降解MT可能存在2条途径, 具体如图 6所示.途径I:如大多数文献中所述(Smet et al., 1998;2010; Gould et al., 1992), MT可在MT氧化酶的作用下形成甲醛和H2S, 随后甲醛和H2S可进一步被氧化.Pseudomonas sp.、Thiobacillus sp.在MT代谢及其中间代谢产物H2S代谢中均有可能起作用.
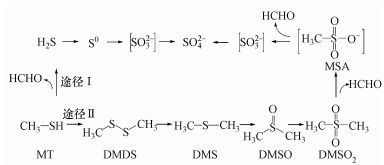 |
| 图 6 MT可能的降解途径 Fig. 6 Proposed pathway for degradation of MT |
由图 4a、4d可知, 微生物在MT向DMDS的氧化过程中起重要作用.混合菌群中菌种较多, 且检测出的中间产物除甲醛、H2S外, 还有DMDS、DMS和DMSO等, 故推测MT的降解存在途径Ⅱ, 即MT依次转化为DMDS、DMS、DMSO和DMSO2, 最后经MSA可生成SO42-.Sipma等(2005) 也认为, 微生物在中性条件下降解MT是通过DMDS这条途径, 并且认为MT转化为DMDS是脱毒过程.原因可能是DMDS与MT相比, 位阻较小, 亲和性较弱(Bosch et al., 2009) .类似的现象在其他硫醇降解过程中也有出现, 如Wang等(2014) 在乙硫醇降解过程中发现二乙基二硫醚先积累后降解;Chen等(2016) 认为丙硫醇降解第一步是丙硫醇转化为二丙基二硫醚;叔丁基硫醇生物降解过程中检测到了2种重要的中间物质, 其中之一就是二叔基二硫醚(Karthikeyan et al., 2012) ;菌株CAN55在降解癸硫醇过程中, 通过GC-MC也检测到了双癸基二硫醚的存在(Chebbi et al., 2014) .所以在途径Ⅱ中, 认为MT代谢第一步就是S—H键断裂生成S—S键, MT转化为DMDS;而后, DMDS可转化为DMS (Liang et al., 2014) ;接着DMS可在异养菌, 如Pseudomonas. sp.、Acinetobacter sp.等的作用下, 转化为DMSO和DMSO2;DMSO2经甲基磺酸(MSA)可生成亚硫酸, 在该过程中Pseudomonas属细菌可能起了重要作用.已证实Pseudomonas putida DS1(Endoh et al., 2003) 中的sfnG和ssuD基因分别与DMSO2脱甲基生成MSA及MSA脱甲基生成亚硫酸两部分降解有关.
4 结论(Conclusions)1) 从处理甲硫醚(DMS)和丙硫醇(PT)混合废气的生物滴滤塔中分离出一组能够有效降解甲硫醇(MT)的混合菌群, 经20代传代培养, MT去除率仍保持在100%.30 ℃、pH=7.0是该混合菌群较为适宜的生长和降解条件;添加酵母膏(YE), 该混合菌群降解20 mg·L-1的MT所需时间缩短10 h.
2) 混合菌群中优势菌属主要有Pseudomonas sp.、Thiobacillus sp.和Acinetobacter sp., 所占比例分别为33.78%、21.91%和17.01%
3) 经测定MT降解产物, 推断MT主要代谢途径有2条:①MT经甲醛、H2S, 直接氧化;②MT依次转化为DMDS、DMS、DMSO和DMSO2, 最后经MSA降解.
| [${referVo.labelOrder}] | Bosch P L F V D, Graaff M D, Fortuny-Picornell M, et al. 2009. Inhibition of microbiological sulfide oxidation by methanethiol and dimethyl polysulfides at natron-alkaline conditions[J]. Applied Microbiology & Biotechnology, 83(3): 579–587. |
| [${referVo.labelOrder}] | Bashkova S, Bagreev A, Bandosz T J. 2002. Adsorption of methyl mercaptan on activated carbons[J]. Environmental Science & Technology, 36(12): 2777–2782. |
| [${referVo.labelOrder}] | Cáceres M, Silva J, Morales M, et al. 2012. Kinetics of the bio-oxidation of volatile reduced sulphur compounds in a biotrickling filter[J]. Bioresour Technol, 118: 243–248. DOI:10.1016/j.biortech.2012.04.039 |
| [${referVo.labelOrder}] | Chan A A. 2006. Attempted biofiltration of reduced sulphur compoundsfrom a pulp and paper mill in Northern Sweden[J]. Environmental Progress, 25(2): 152–160. DOI:10.1002/(ISSN)1547-5921 |
| [${referVo.labelOrder}] | Chebbi A, Mnif S, Mhiri N, et al. 2014. A moderately thermophilic and mercaptan-degrading Bacillus licheniformis strain CAN55 isolated from gas-washing wastewaters of the phosphate industry,Tunisia[J]. International Biodeterioration & Biodegradation, 94(94): 207–213. |
| [${referVo.labelOrder}] | Chen D Z, Sun Y M, Han L M, et al. 2016. A newly isolated Pseudomonas putida S-1 strain for batch-mode-propanethiol degradation and continuous treatment of propanethiol-containing waste gas[J]. Journal of Hazardous Materials, 302: 232–240. DOI:10.1016/j.jhazmat.2015.09.063 |
| [${referVo.labelOrder}] | Cho K S, Zhang L, Hirai M, et al. 1991. Removal characteristics of hydrogen sulphide and methanethiol by Thiobacillus sp[J]. isolated from peat in biological deodorization[J].Journal of Fermentation & Bioengineering, 71(1): 44–49. |
| [${referVo.labelOrder}] | Couvert A, Charron I, Laplanche A, et al. 2006. Treatment of odorous sulphur compounds by chemical scrubbing with hydrogen peroxide-Application to a laboratory plant[J]. Chemical Engineering Science, 61(22): 7240–7248. DOI:10.1016/j.ces.2006.07.030 |
| [${referVo.labelOrder}] | De Bont J A M. 1981. Dimethyl sulphoxide and dimethyl sulphide as a carbon,sulphur and energy source for growth of Hyphomicrobium S[J]. Microbiology, 127(2): 315–323. DOI:10.1099/00221287-127-2-315 |
| [${referVo.labelOrder}] | Endoh T, Habe H, Yoshida T, et al. 2003. A CysB-regulated and sigma54-dependent regulator,SfnR,is essential for dimethyl sulfone metabolism of Pseudomonas putida strain DS1[J]. Microbiology, 149(4): 991–1000. DOI:10.1099/mic.0.26031-0 |
| [${referVo.labelOrder}] | Gould W D, Kanagawa T, Gould W D. 1992. Purification and properties of methyl mercaptan oxidase from Thiobacillus thioparus TK-m[J]. Journal of General Microbiology, 138(1): 217–221. DOI:10.1099/00221287-138-1-217 |
| [${referVo.labelOrder}] | Endoh T, Kasuga K, Horinouchi M, et al. 2003. Characterization and identification of genes essential for dimethyl sulfide utilization in Pseudomonas putida strain DS1[J]. Applied Microbiology and Biotechnology, 62(1): 83–91. DOI:10.1007/s00253-003-1233-7 |
| [${referVo.labelOrder}] | Horinouchi M, Kasuga K, Nojiri H, et al. 1997. Cloning and characterization of genes encoding an enzyme which oxidizes dimethyl sulfide in Acinetobacter sp[J]. strain 20B[J].Fems Microbiology Letters, 155(1): 99–105. DOI:10.1111/j.1574-6968.1997.tb12692.x |
| [${referVo.labelOrder}] | Hulea V, Huguet E, Cammarano C, et al. 2014. Conversion of methyl mercaptan and methanol to hydrocarbons over solid acid catalysts-A comparative study[J]. Applied Catalysis B Environmental, 144(2): 547–553. |
| [${referVo.labelOrder}] | Iranpour R, Cox H H J, Fan S, et al. 2005. Short-term and long-term effects of increasing temperatures on the stability and the production of volatile sulfur compounds in full -scale thermophilic anaerobic digesters[J]. Biotechnology and Bioengineering, 91(2): 199–212. DOI:10.1002/(ISSN)1097-0290 |
| [${referVo.labelOrder}] | Ito T, Miyaji T, Nakagawa T, et al. 2007. Degradation of dimethyl disulfide by Pseudomonas fluorescens strain 76[J]. Agricultural and Biological Chemistry, 71(2): 366–370. |
| [${referVo.labelOrder}] | Karnofski M A. 1975. Odor generation in the kraft process[J]. Journal of Chemical Education, 52(8): 490. DOI:10.1021/ed052p490 |
| [${referVo.labelOrder}] | Lebrero R, Gondim A C. 2014. Comparative assessment of a biofilter,a biotrickling filter and a hollow fiber membrane bioreactor for odor treatment in wastewater treatment plants[J]. Water Research, 49(1): 339–350. |
| [${referVo.labelOrder}] | Liang Z, An T, Li G, et al. 2014. Aerobic biodegradation of odorous dimethyl disulfide in aqueous medium by isolated Bacillus cereus GIGAN2 and identification of transformation intermediates[J]. Bioresource Technology, 175: 563–568. |
| [${referVo.labelOrder}] | 刘洪霞, 朱润晔, 欧阳杜娟, 等. 2013. 二氯甲烷降解菌Methylobacterium rhodesianum H13的分离鉴定及降解特性研究[J]. 环境科学, 2013, 34(9): 3613–3619. |
| [${referVo.labelOrder}] | 林琳. 2000. 生物法去除含甲硫醇恶臭气体的机理[J]. 辽宁城乡环境科技, 2000, 20(4): 7–8. |
| [${referVo.labelOrder}] | 李政, 赵朝成, 张云波, 等. 2011. 耐热石油降解混合菌群的降解性能研究[J]. 化学与生物工程, 2011, 28(12): 37–42. DOI:10.3969/j.issn.1672-5425.2011.12.010 |
| [${referVo.labelOrder}] | Nagaev A V, Starcev A N. 1988. Isolation and physiological characterization of autotrophic sulphur bacteria oxidizing dimethyl disulphide as sole source of energy[J]. Microbiology, 134(6): 1407–1417. DOI:10.1099/00221287-134-6-1407 |
| [${referVo.labelOrder}] | Park S J, Cho K S, Hirai M, et al. 1993. Removability of malodorous gases from a night soil treatment plant by a pilot-scale peat biofilter inoculated with thiobacillus thioparus DW44[J]. Journal of Fermentation & Bioengineering, 76(1): 55–59. |
| [${referVo.labelOrder}] | Pol A, Camp H J M O D, Mees S G M, et al. 1994. Isolation of a dimethylsulfide-utilizing Hyphomicrobium species and its application in biofiltration of polluted air[J]. Biodegradation, 5(2): 105–112. DOI:10.1007/BF00700635 |
| [${referVo.labelOrder}] | Karthikeyan R, Hutchinson S L L, Erickson L E. 2012. Biodegradation of tertiary butyl mercaptan in water[J]. Journal of Bioremediation & Biodegradation. |
| [${referVo.labelOrder}] | Rosenfeld P E, Henry C L, Bennett D. 2001. Wastewater dewatering polymer affect on biosolids odor emissions and microbial activity[J]. Water Environment Research, 73(73): 363–367. |
| [${referVo.labelOrder}] | Shu C H, Chen C K. 2009. Enhanced removal of dimethyl sulfide from a synthetic waste gas stream using a bioreactor inoculated with Microbacterium sp[J]. NTUT26 and Pseudomonas putida[J].Journal of Industrial Microbiology & Biotechnology, 36(1): 95–104. |
| [${referVo.labelOrder}] | Sipma J, Svitelskaya A, Mark B V D, et al. 2005. Potentials of biological oxidation processes for the treatment of spent sulfidic caustics containing thiols[J]. Water Research, 38(20): 4331–4340. |
| [${referVo.labelOrder}] | Sivelä S, Sundman V. 1975. Demonstration of thiobacillus-type bacteria,which utilize methyl sulphides[J]. Archives of Microbiology, 103(1): 303–304. DOI:10.1007/BF00436365 |
| [${referVo.labelOrder}] | Smet E, Van Langenhove H. 1998. Abatement of volatile organic sulfur compounds in odorous emissions from the bio-industry[J]. Biodegradation, 9(3/4): 273–284. DOI:10.1023/A:1008281609966 |
| [${referVo.labelOrder}] | Smet E, Lens P, Van Langenhove H. 1998. Treatment of waste gases contaminated with odorous sulfur compounds[J]. Critical Reviews in Environmental Science and Technology, 28(1): 89–117. DOI:10.1080/10643389891254179 |
| [${referVo.labelOrder}] | Suylen G M H, Stefess G C, Kuenen J G. 1986. Chemolithotrophic potential of a Hyphomicrobium species,capable of growth on methylated sulphur compounds[J]. Archives of Microbiology, 146(2): 192–198. DOI:10.1007/BF00402350 |
| [${referVo.labelOrder}] | Tanji Y, Kanagawa T, Mikami E. 1986. Removal of dimethyl sulfide,methyl mercaptan,and hydrogen sulfide by immobilized Thiobacillus thioparus TK-m[J]. Journal of Fermentation & Bioengineering, 67(4): 280–285. |
| [${referVo.labelOrder}] | van Leerdam R C, Bonilla-Salinas M, de Bok F Am, et al. 2008. Anaerobic methanethiol degradation and methanogenic community analysis in an alkaline (pH 10) biological process for liquefied petroleum gas desulfurization[J]. Biotechnology and Bioengineering, 101(4): 691–701. DOI:10.1002/bit.21933 |
| [${referVo.labelOrder}] | Wang X, Wu C, Liu N, et al. 2015. Degradation of ethyl mercaptan and its major intermediate diethyl disulfide by Pseudomonas sp[J]. strain WL2[J].Applied Microbiology and Biotechnology, 99(7): 3211–3220. DOI:10.1007/s00253-014-6208-3 |
| [${referVo.labelOrder}] | 王艳青, 张劲松, 周集体, 等. 2009. 对氨基苯磺酸降解菌的分离鉴定及降解特性研究[J]. 环境科学, 2009, 30(4): 1193–1198. |
| [${referVo.labelOrder}] | 肖巧巧, 尹华, 叶锦韶, 等. 2014. 一株微囊藻毒素-LR降解菌的降解特性[J]. 环境化学, 2014, 33(9): 1594–1600. DOI:10.7524/j.issn.0254-6108.2014.09.010 |
| [${referVo.labelOrder}] | 杨丽卿.2013.偶氮染料派脱色菌的选育及脱色效果研究[D].长春:吉林大学 |
| [${referVo.labelOrder}] | Zhang L, Hirai M, Shoda M. 1992. Removal characteristics of dimethyl sulfide by a mixture of Hyphomicrobium sp[J]. I55 and Pseudomonas acidovorans DMR-11[J].Journal of Fermentation & Bioengineering, 74(3): 174–178. |
| [${referVo.labelOrder}] | Zhang L, Kuniyoshi I, Hirai M, et al. 1991. Oxidation of dimethyl sulfide by Pseudomonas acidovorans DMR-11 isolated from peat biofilter[J]. Biotechnology Letters, 13(3): 223–228. DOI:10.1007/BF01025822 |
| [${referVo.labelOrder}] | 朱婷婷, 倪晋仁. 2012. 不同碳源对苯并[a]芘降解菌生长和降解性能影响的研究[J]. 北京大学学报(自然科学版), 2012, 48(2): 343–346. |
| [${referVo.labelOrder}] | 张锡辉, BaipaiR. 2000. 以关键酶为基础共代谢模型的建立—以甲烷细菌共代谢三氯乙烯为例[J]. 环境科学学报, 2000, 20(5): 558–562. |
 2017, Vol. 37
2017, Vol. 37