2. 工业聚集区污染控制与生态修复教育部重点实验室, 广州 510006
2. The Key Lab of Pollution Control and Ecosystem Restoration in Industry Clusters, Ministry of Education, Guangzhou 510006
现如今我们生活在一个塑料的时代, 全球每年塑料的产量达到了成百上千万吨(Thompson et al., 2004; Horton et al., 2007).在过去几十年里, 塑料碎片在环境中广泛存在并大量富集(Andrady et al., 2011; Lönnstedt et al., 2016).受气候等环境因素的影响, 大块的塑料随时间逐渐被分解成小碎片, 一些碎片甚至达到了微米级别, 粒径小于5 mm的塑料定义为微塑料(Barnes et al., 2009; Arthur et al., 2009; Weinstein et al., 2016).由于人类活动频繁, 近岸环境中微塑料污染问题更为显著(Wright et al., 2013; Browne et al., 2010), 以珠江口为例, 近岸微塑料的数量密度可达到(347.0±205.9) n·m-2(赵世烨, 2017).由于微塑料的尺寸较小, 极易被生物摄入, 通过食物链在各层级生物体内富集(Juliana et al., 2014), 现已发现在很多生物的肌肉、胃、肠道中均有微塑料颗粒存在(Oliveira et al., 2012; Wright et al., 2013).微塑料的富集对周围的生态系统都造成了潜在的危害.目前, 有关微塑料的研究更多集中于微塑料在生物体内的积累, 如在河口区采集的23%野生海鲶(Cathorops spp.和Sviades herzbergii)和7.9%的石首鱼(Stellifer spp.)体内都检测到有微塑料(Possatto et al., 2011; Dantas et al., 2012), 但有关微塑料对微生物生态影响的研究还较少, 已有研究表明塑料在海洋环境里能作为载体供微生物附着生长, 由于塑料的疏水性, 其表面可有利于微生物生物膜的形成(Goldstein et al., 2012; Zettler et al., 2013).
近岸环境中存在着丰富的微生物, 其活动是环境污染和生态系统之间的关键环节(Rummel et al., 2017).而石油烃类是近岸环境中的主要污染物之一, 微生物在降解石油及其组分中起着重要作用(钟磊等, 2010).由于塑料微粒比表面积较大, 在环境中微塑料能吸附一定量的持久性有机污染物(POPs), 如多环芳烃等(Herzke et al., 2016), 那么微塑料可能会影响微生物对石油烃组分的降解.因此, 本研究目的主要包括①研究微塑料进入到近岸环境中对多环芳烃降解能力的影响; ②进一步考察微塑料对多环芳烃降解过程中微生物菌群结构的影响.为此, 本文从广州市南沙区珠江入海口受石油污染的水体中采集样品, 选用石油组分中多环芳烃的典型代表物——菲, 驯化得到菲降解菌群, 考察微塑料对降解菌群结构及其降解能力的影响, 旨在探究微塑料对近海岸石油污染降解的影响, 为进一步对近岸污染环境的修复提供理论依据.
2 材料与方法(Materials and methods) 2.1 试验材料菲(Phenanthrene)购买自Sigma-Aldrich公司, 纯度为98%, 甲醇、丙酮为分析纯.选择环境中广泛存在的聚氯乙烯(PVC)为实验微塑料(Bakir et al., 2014), 购自阿拉丁试剂(上海)有限公司, 其粒径在200~250 μm之间, 每次实验前置于超净台中用紫外灭菌处理.
无机盐培养液(MSM):磷酸盐缓冲液(27.60 g·L-1 Na2HPO4·2H2O, 35.60 g·L-1 NaH2PO4·2H2O); 3 mL MgSO4溶液(22.5 g·L-1); 1.0 mL FeCl3溶液(0.25 g·L-1); 1.0 mL CaCl2溶液(36.4 g·L-1); 1.0 mL微量元素溶液(MnSO4.H2O, 39.9 mg·L-1; ZnSO4·H2O, 42.8 mg·L-1; (NH4)6Mo7O24.4H2O, 34.7 mg·L-1); pH约为7.2, NaCl溶液调节培养基盐度, 盐度为1.5%.
含有菲的无机盐培养液:以丙酮配制20 g·L-1的菲溶液, 取一定量的菲溶液, 置于灭菌的三角瓶中, 待丙酮挥发完毕加入灭菌的无机盐基础培养液.
固体培养基:1.8 g营养肉汤, 2.0 g琼脂, 100 mL蒸馏水.
2.2 菲降解菌群的驯化实验用样品来自珠江入海口广州南沙区受石油污染的近岸水体.
取50 mL所采集的样品加入到100 mL含有菲的无机盐培养基中, 放入30 ℃摇床中恒温避光振荡培养, 约6 d后, 观察到三角瓶中液体变浑浊, 肉眼已观察不到溶液中的菲.随后移取10 mL上述富集的菌液到新配制的90 mL的已灭菌的含有菲的无机盐培养液中, 如此重复富集培养8次, 得到稳定的富集菌群.最后一次取2 mL的菌液到18 mL含有菲(40 mg·L-1)的无机盐培养液中, 测定6 d后混合菌系的菲的降解率, 对照组为不加菌的菲无机盐培养液.
2.3 微塑料对菌群MB1降解效果的影响向含有40 mg·L-1菲的MSM培养基中加入2 mL的菌液, 设定3个实验组, 一个空白组和一个对照组.实验组微塑料的浓度分别为100、200和400 mg·L-1;以灭菌含有等量菲的MSM培养基作为空白组;以加入同等量菌液而不加微塑料的含有菲的MSM培养基作为对照组.将所有样品放入30 ℃摇床中避光振荡培养, 设立多组平行样, 分别在第3 d和第6 d时, 每组牺牲3个平行样测定样品中剩余菲的量.
2.4 SEM分析利用扫描电镜SEM(Car Zeiss Jena Merlin)观察微塑料表面在有无菌群存在时的变化情况, 添加微塑料的实验组中微塑料浓度为400 mg·L-1.在一定时间间隔, 样品被取出并用磷酸盐缓冲溶液冲洗后, 用戊二醛预先固定12 h后, 利用一系列浓度梯度的乙醇脱水干燥(30%、50%、75%、95%、100%的乙醇每次脱水10~15 min), 最后用100%的叔丁醇替换乙醇后放进冷冻干燥机中冷冻干燥2 d.所有样品喷金后放进扫描电镜下观察分析.
2.5 微塑料对菌群MB1群落结构变化的影响实验设置在有400 mg·L-1微塑料存在的菌液为实验组, 同时以不加微塑料的为对照组.分别在第0、3和第6 d时取出一定量菌液, 将样品送至广州美格基因科技有限公司对菌群群落进行分析.分析方法为先对样品进行DNA提取, 以基因组DNA为模板, 使用16S V4区引物(515F和806R)进行PCR扩增, 利用GeneTools Analysis Software(Version4.03.06.0, SynGene)对PCR产物进行浓度对比后, 将各PCR产物进行混合;使用EZNA Gel Extraction Kit (Omega, USA)凝胶回收试剂盒回收PCR混合产物, TE缓冲液洗脱回收目标DNA片段;按照NEBNext® UltraTM DNA Library Prep Kit for Illumina® (New England Biolabs, USA)标准流程进行建库操作;使用Illumina Hiseq2500平台对构建的扩增子文库进行PE250测序.
2.6 菲降解率的测定样品取出后加入适量的甲醇, 经超声处理后, 待瓶中残留的菲溶解后, 将溶液转移到50 mL容量瓶中并用甲醇定容.随后移取1.5 mL溶液过0.22 μm滤膜后用Agilent高效液相HPLC测定菲的剩余量.降解效率的计算公式如下:

|
(1) |
式中, A0表示空白对照组的峰面积值, A表示降解系统的峰面积值.
3 结果与讨论(Results and discussion) 3.1 富集培养的菲降解菌群将污染水样接种在含有菲的无机盐培养基中, 经过从低浓度到高浓度的多次富集、转接、培养, 最终获得能降解菲的混合微生物菌群MB1.菌群MB1培养6 d后, 对菲(40 mg·L-1)的降解率可达85%左右.采用Illumina序列分析菌群群落结构可知, 菌群中的相对优势菌主要为Alteromonas sp.、Prochlorococcus sp.和小红卵菌Rhodovulum sp..Jin等(2012)的研究中曾报道过在受石油污染的近岸沉积物中, Alteromona对多环芳烃的生物降解起到关键的作用.
3.2 微塑料对菲降解菌群降解菲的影响添加不同质量浓度的微塑料, 分别在第3 d和第6 d时取样测定了菌群对菲的降解率, 其结果如图 1所示.在第3 d时, 添加了微塑料的体系中菌群对菲的降解率会略高于对照组.到第6 d时, 添加了微塑料的实验组中菲都基本被完全去除, 而对照组中菌群对菲的降解效率为80%左右, 表明微塑料能在一定程度上促进菌群对菲的降解.
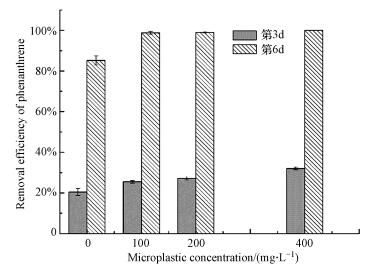 |
| 图 1 微塑料对菌群MB1降解菲的影响 Fig. 1 Effect of microplastic on phenanthrene biodegradation by bacterial consortium MB1 |
SEM图(图 2)显示, 添加质量浓度为400 mg·L-1微塑料培养2 d后, 能观察到有菲晶体吸附在塑料表面(图 2a), Bakir等(2014)也曾报道菲能吸附在塑料-聚氯乙烯颗粒上.随着培养时间延长, 在第4 d时, 在塑料表面已观察不到有菲吸附, 说明微塑料上的菲被菌群降解利用, 此外, 有部分菌附着在塑料上(图 2b, 如白色箭头所示).到第6 d时, 相比于对照组中存在的少量丝状物(图 2d), 加了微塑料的组会分泌更多的丝状物质(图 2c), 推测其可能为微生物分泌的胞外分泌物, 胞外分泌物一般包括多糖、蛋白质、脂质等(Stewart et al., 2013).这些丝状物不仅将菌和菌相连, 还连接了塑料和菌, 使菌能紧紧附着在塑料上(图 2b, 如白色箭头所示).当丝状物连接了菌与微塑料时, 可能会有利于菌群利用塑料表面的菲.此外生物膜能为微生物在体系中生长提供一系列的有利条件, 其中包括能为细菌形成稳定的菌团以及基因的水平交换提供可能, 此外还有利于细菌对营养物质的吸收, 并且能够帮助微生物抵御有毒物质和干燥的环境(Flemming, 1998).
 |
| 图 2 SEM图(在400 mg·L-1微塑料存在情况下分别培养了2 d(a), 4 d(b), 6 d(c)的细胞;(d)培养了6 d的未加微塑料的对照组细胞) Fig. 2 SEM images showing(a, b and c) cells in the presence of 400 mg·L-1 microplastic with incubation time of 2, 4, 6 days, respectively; (d) control cells with incubation time of 6 days |
采用Illumina序列进一步分析微塑料对菲降解菌群群落结构变化的影响, 比较分析添加了400 mg·L-1微塑料对菲降解过程中, 第0、3和6 d时的实验组及未加微塑料的对照组菌群的群落结构组成(图 3).在第3 d时, 实验组和对照组中群落结构中的优势菌差异不大, 两组中Synechococcus均为优势菌, 有研究称Synechococcus sp. PR-6能降解菲, 并在降解菲的过程中首次发现生成了菲的代谢产物1-甲氧基菲烷(Ouyang, 2017).到第6 d, 菌群结构发生了明显的变化, 对照组(J6)中的优势菌为小红卵菌Rhodovulum, 而在添加了400 mg·L-1微塑料实验组(M6)里, 其优势菌为Glaciecola.第3 d时, 实验组(M3)中Glaciecola的相对丰度为1.02%, 在第6 d时Glaciecola相对丰度增加到36.74%, 在Krolicka(2017)的实验中发现添有石油的水槽里Glaciecola的相对丰度也增加了近30倍;此外, 相比于对照组(J3和J6)中的Glaciecola相对丰度无明显变化, 实验组中Glaciecola相对丰度的大大增加表明菌Glaciecola不仅能降解石油烃, 还可能对微塑料起到了降解作用.Morohoshi(2018)在对微塑料的降解的研究中称, 在聚(3-羟基丁酸-3-羟基乙酸酯)(PHBH)表面观察到有大量的生物膜, 且此时大部分的PHBH膜都已被降解, 而在生物膜中占主导的菌与Glaciecola具有极高的相似性.另外, 从图中可知在第6 d时相比只有菌的对照组, 添加了微塑料的实验组中Oleibacter的相对丰度也有所提高, Maki(2011)报道了其筛选得到的菌Oleibacter marinus可降解石油脂肪烃类.说明Glaciecola和Oleibacter可能是可附着在微塑料上生长的一类细菌.菌群结构的分析进一步说明添加微塑料可改变微生物降解菌的结构, 同时可能对降解菲能起到一定程度的促进作用.
 |
| 图 3 物种在属水平上的相对丰度分布图(横坐标中J代表只有菌的对照组, M代表加了400 mg·L-1微塑料的实验组, 数字代表培养的时间) Fig. 3 Relative abundance distribution of species in the genus level(J represents control group, M represents bacteria in the presence of 400 mg·L-1 microplastic, the number represents time) |
1) 石油污染的近岸环境中, 微塑料的进入可能促进了可降解多环芳烃的微生物的附着和菲的降解.
2) 添加了微塑料的体系中优势菌为Glaciecola, 而对照组中优势菌为小红卵菌Rhodovulum, 优势种群发生变化, 说明微塑料的添加在一定程度上会改变多环芳烃降解过程中微生物群落结构, 可能进一步影响污染物的降解能力.
Andrady A L. 2011. Microplastics in the marine environment[J]. Marine Pollution Bulletin, 62(8): 1596–1605.
|
Arthur C, Baker J, Bamford H E. 2009. Proceedings of the international research workshop on the occurrence, effects and fate of microplastic marine debris[OL].2018-03-21. http://www.researchgate.net/publication/309563149
|
Bakir A, Rowland S J, Thompson R C, et al. 2014. Transport of persistent organic pollutants by microplastics in estuarine conditions[J]. Estuarine Coastal & Shelf Science, 140(3): 14–21.
|
Barnes D K, Galgani F, Thompson R C, et al. 2009. Accumulation and fragmentation of plastic debris in global environments[J]. Philosophical Transactions of the Royal Society of London B:Biological Sciences, 364(1526): 1985–1998.
DOI:10.1098/rstb.2008.0205
|
Browne M A, Galloway T S, Thompson R C. 2010. Spatial patterns of plastic debris along estuarine shorelines[J]. Environment Science & Technology, 44: 3404–3409.
|
Dantas D V, Barletta M, Costa M F. 2012. The seasonal and spatial patterns of ingestion of polyfilament nylon fragments by estuarine drums[J]. Environmental Science and Pollution Reasearch, 19(2): 600–606.
DOI:10.1007/s11356-011-0579-0
|
Flemming H C. 1998. Relevance of biofilms for the biodeterioration of surfaces of polymeric materials[J]. Polymer Degradation and Stability, 59(1/3): 309–315.
|
Goldstein M C, Rosenberg M, Cheng L. 2012. Increased oceanic microplastic debris enhances oviposition in an endemic pelagic insect[J]. Biology Letters, 8(5): 817–820.
DOI:10.1098/rsbl.2012.0298
|
Herzke D, Ankernilssen T, Nøst T H, G tsch A, et al. 2016. Negligible impact of ingested microplastics on tissue concentrations of persistent organic pollutants in Northern Fulmars off coastal Norway[J]. Environmental Science & Technology, 50(4): 1924–1933.
|
Horton A A, Walton A, Spurgeon D J, et al. 2007. Microplastics in freshwater and terrestrial environments:Evaluating the current understanding to identify the knowledge gaps and future research priorities[J]. Science of the Total Environment, 586: 127–141.
|
Jin H M, Kim J M, Lee H J, et al. 2012. Alteromonas as a key agent of polycyclic aromatic hydrocarbon biodegradation in crude oil-contaminated coastal sediment[J]. Environmental Science & Technology, 46(14): 7731–7740.
|
Juliana A, Ivar do Sul, Monica F, et al. 2014. The present and future of microplastic pollution in the marine environment[J]. Environmental Pollution, 185: 352–364.
DOI:10.1016/j.envpol.2013.10.036
|
Krolicka A, Boccadoro C, Nilsen M M, et al. 2017. Capturing early changes in the marine bacterial community as a result of crude oil pollution in a mesocosm experiment[J]. Microbes and Environments, 32(4): 358–366.
DOI:10.1264/jsme2.ME17082
|
Lönnstedt O M, Ekl v P. 2016. Environmentally relevant concentrations of microplastic particles influence larval fish ecology[J]. Science, 352(6290): 1213–1216.
DOI:10.1126/science.aad8828
|
Maki T, Motoyuki O, Ariani H, et al. 2011. Oleibacter marinus gen. nov., sp. nov., a bacterium that degrades petroleum aliphatic hydrocarbons in a tropical marine environment[J]. International Journal of Systematic and Evolutionary Microbiology, 61: 375–380.
DOI:10.1099/ijs.0.018671-0
|
Morohoshi T, Tomohiro, Ogata K, et al. 2018. Molecular characterization of the bacterial community in biofilms for degradation of Poly (3-Hydroxybutyrate-co-3-Hydroxyhexanoate) films in seawater[J]. Microbes and Environments, 33(1): 19–25.
DOI:10.1264/jsme2.ME17052
|
Oliveira M, Ribeiro A, Guilhermino L. 2012. Effects of exposure to microplastics and PAHs on microalgae Rhodomonas baltica and Tetraselmis chuii[J]. Comparative Biochemistry and Physiology, Part A, 163: S19–S20.
|
Ouyang J. 2017. Phenanthrene Pathway Map[OL]. 2018-03-21.http://eawag-bbd.ethz.ch/pha/pha_map.html
|
Possatto F E, Barletta M, Costa M F, et al. 2011. Plastic debris ingestion by marine catfish:An unexpected fisheries impact[J]. Marine Pollution Bulletin, 62(5): 1098–1102.
DOI:10.1016/j.marpolbul.2011.01.036
|
Rummel C D, Jahake A, Gorokhova E, et al. 2017. Impacts of biofilm formation on the fate potential effects of microplastic in the aquatic environment[J]. Environmental Science & Technology Letters, 4(7): 258–267.
|
Stewart T J, Traber J, Kroll A, et al. 2013. Characterization of extracellular polymeric substances (EPS) from periphyton using liquid chromatography-organic carbon detection-organic nitrogen detection (LC-OCD-OND)[J]. Environmental Science Pollution Research, 20: 3214–3223.
DOI:10.1007/s11356-012-1228-y
|
Thompson R C, Olsen Y, Mitchell R P, et al. 2004. Lost at sea:where is all the plastic?[J]. Science, 304(5672): 838–838.
DOI:10.1126/science.1094559
|
Weinstein J E, Crocker B K, Gray A D. 2016. From macroplastic to microplastic:Degradation of high-density polyethylene, polypropylene, and polystyrene in a salt marsh habitat[J]. Environmental Toxicology& Chemistry, 35(7): 1632–1640.
|
Wright S L, Thompson R C, Galloway T S. 2013. The physical impacts of microplastics on marine organisms:a review[J]. Environmental Pollution, 178: 483–492.
|
Zettler E R, Mincer T J, Linda A, et al. 2013. Life in the "plastisphere":microbial communities on plastic marine debris[J]. Environmental Science & Technology, 47: 7137–7146.
|
赵世烨. 2017.中国部分河口微塑料的赋存特征及海洋雪中微塑料分析方法研究[D].上海: 华东师范大学. 51-66
|
钟磊, 周立祥, 王世. 2010. 菲降解菌的分离鉴定及其在污染土壤生物修复中的应用[J]. 农业环境科学学报, 2010, 29(3): 465–470.
|
 2018, Vol. 38
2018, Vol. 38


