2. 中国科学院大学, 北京 100049
2. University of Chinese Academy of Sciences, Beijing 100049
厌氧氨氧化(Anaerobic ammonium oxidizing, Anammox)工艺是在厌氧或缺氧条件下, 厌氧氨氧化菌利用NO2-为电子受体氧化NH4+为N2的一种化能自养型脱氮工艺(Wr et al., 2007).Anammox工艺因具有脱氮效率高、污泥产量低、无需外加碳源等优势而倍受欢迎, 已被用于实际工程中(Jin et al., 2012a;2012b;Zhang et al., 2013).但Anammox菌具有生长速率慢、倍增时间长、环境敏感度高等缺陷(Jin et al., 2012b;Wr et al., 2007), 增加了Anammox工艺受损后的恢复难度.实际废水, 尤其是工业废水中, 化学成分复杂(He et al., 2016;Li et al., 2017b;Zhang et al., 2013), 氮浓度较高, 其中的NO2--N和NH4+-N虽为Anammox菌的生长基质, 但同时也是毒性物质, 极易影响Anammox菌的生长代谢, 进而威胁Anammox工艺的运行(Li et al., 2017a;2012b).研究表明, NO2--N对Anammox工艺的影响高于NH4+-N(Puyol et al., 2014;Carvajalarroyo et al., 2015), NH4+-N浓度低于1000 mg · L -1时不会抑制Anammox菌活性, 而NO2--N浓度高于280 mg · L -1时便会产生抑制, 因此, 基质抑制尤其是NO2--N抑制是Anammox工艺广泛应用的瓶颈之一(Dapena-Mora et al., 2007;Isaka et al., 2007;Wang et al., 2016;Strous, 1999).因此, 高浓度基质抑制后Anammox菌的活性恢复及其恢复策略研究将有助于Anammox工艺的推广应用.
胞外聚合物(Extracellular Polymeric Substances, EPS)作为微生物新陈代谢和细胞自溶分泌的产物, 是一类附着于细胞壁表面的有机大分子多聚物, 主要由结合型EPS构成, 其又分为紧密型(TB-EPS)和松散型(LB-EPS)两种, 分别由内到外包埋微生物, 以维持细胞结构和功能的完整性(Badireddy et al., 2010;Sheng et al., 2010;Zhu et al., 2009).EPS主要由蛋白质(Protein, PN)和多糖(Polysaccharide, PS)构成, 其中, PS是由中性的和带电的糖苷键构成的异质多糖体, 以各种结合力与基质中的生物分子构成EPS的三维网状结构, 而PN则包含有胞外酶、EPS修饰酶及结构蛋白等, 被固定于PS中, 降解基质中聚合物并修饰EPS结构, 二者相互作用以促进微生物间的物质转换和能量传递, 增强微生物的耐冲击能力并维持其酶活性及功能特性(Flemming et al., 2010).PN和PS浓度易受水质条件、反应器运行方式及优势菌种代谢水平等因素的影响(Hou et al., 2015;Yin et al., 2015;Zhu et al., 2009), 可很好地反映Anammox菌的活性恢复情况.本研究通过考察受基质抑制后的Anammox菌在活性恢复过程中, 其脱氮性能、EPS组分及Anammox菌丰度的变化, 探究升流式厌氧反应器中Anammox菌的活性恢复特性, 以期为Anammox工艺恢复的机制研究及实际应用提供理论与技术支持.
2 材料和方法(Materials and methods) 2.1 试验装置试验采用升流式厌氧氨氧化反应器, 具体如图 1所示.反应器制作材料为有机玻璃, 有效容积1.5 L, 顶部设有三相分离装置和溢流堰, 处理水经溢流堰排出, 反应器内部挂有辫帘式填料, 便于形成生物膜, 上部设有回流装置(回流比为4), 促进反应器内部循环, 并防止进水口堵塞.反应器整体置于恒温(35±2) ℃水浴箱中, 避光运行, 进水pH经0.1 mol · L-1盐酸调节至7.5左右, 维持Anammox菌适宜的生长环境.
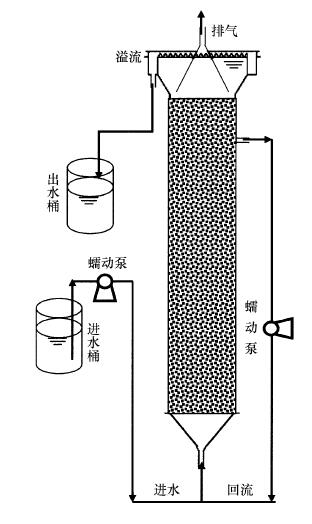 |
| 图 1 实验装置示意图 Fig. 1 Schematic diagram of the experimental device |
试验用水为人工配制的模拟废水, 具体组成见表 1.其中, 微量元素成分为(g · L-1):EDTA 20、ZnSO4 · 7H2O 0.43、CoCl2 · 6H2O 0.24、MnCl2 · 4H2O 0.99、CuSO4 · 5H2O 0.25、NaMoO4 · 2H2O 0.043、NiCl2 · 6H2O 0.20、KH2PO4 20、H3BO3 0.014.于进水桶和出水口处, 分别用离心管收集水样, 经0.45 μm滤膜过滤后分别采用纳氏试剂法、N-(1-萘基)乙二胺分光光度法和紫外分光光度法测定样品中的NH4+-N、NO2--N和NO3--N (Hendrickx et al., 2014).pH采用便携式pH计(FG2-FK, METTLER TOLEDO, USA)测定;污泥样品MLSS、MLVSS均按(国家环境保护总局《水和废水监测分析方法》编委会, 2002)的标准方法测定.
| 表 1 模拟废水成分 Table 1 The composition of synthetic wastewater |
本研究试验前反应器已稳定运行1年, 填料中生长砖红色生物膜, 游离的污泥呈红色松散颗粒状.在进水TN浓度(NH4+-N为350 mg · L-1, NO2--N为350 mg · L-1)为700 mg · L-1, HRT为9 h的条件下, 反应器NH4+-N和NO2--N去除率稳定在94%和83%左右.之后因中断反应器回流, 且仍以TN浓度700 mg · L-1的进水运行, 致使进水区域NO2--N浓度由回流时的20.92 mg · L-1剧增为320.24 mg · L-1, 引起基质抑制, 反应器于之后的1周内脱氮性能骤降, NH4+-N和NO2--N去除率分别降至9.67%和8.75%, 泥色呈灰黑色, 略有臭味, 此时, 反应器中MLSS为7.46 g · L-1, MLVSS为3.96 g · L-1.之后试验采用阶段式提高氮负荷的方法恢复反应器中Anammox菌的活性, 分5个阶段进行, TN浓度分别为160、320、500、700和1000 mg · L-1, 具体运行策略见表 2.
| 表 2 反应器各阶段运行参数 Table 2 Reactor operation parameters of each phase |
污泥样品中的EPS采用高速离心与超声组合的方式提取(Yu et al., 2009), 即取适量4 ℃下静置1.5 h的污泥, 经缓冲液(Na3PO4 : NaH2PO4 : NaCl : KCl=2 : 4 : 9 : 1, pH=7.0)重悬至初始体积后, 4 ℃下2000 g离心15 min, 弃去上清液, 底部沉淀物再次重悬, 并离心(2000 g, 15 min), 此上清液即为LB-EPS;重复底部沉淀物的重悬步骤, 经20 kHz、480 W超声破碎仪(Scienta-IID, China)超声10 min (工作1 s-停止2 s)后, 4 ℃下2000 g离心20 min, 上清液即为TB-EPS.LB-EPS和TB-EPS经0.45 μm聚四氟乙烯滤膜过滤后进行蛋白质与多糖的测定.EPS中蛋白质采用BCA蛋白浓度测定试剂盒(P0010S, 碧云天, 中国)测定, 以牛血清蛋白为标准物质(欧清梅, 2015);多糖采用蒽酮-硫酸法测定, 以葡萄糖为标准物质(张俊珂等, 2016).
2.4 DNA提取与实时定量PCR称取500 mg污泥样品, 使用FastDNATM SPIN Kit for Soil(LLC, MP Biomedicals, USA)提取试剂盒按其操作步骤提取污泥样品中总DNA.实时定量PCR实验采用Roche LightCycler®480 Ⅱ(Roche Diagnostics Ltd, Rotkreuz, Swltzerland)实时荧光定量系统进行, 反应采用20 μL体系, 具体配置为:SYBR Green Ⅰ Master(LightCycler®480, mannheim, Germany)10 μL, 前后引物各0.8 μL, 质粒或DNA样品1 μL, 去离子水7.4 μL.其中, 全细菌定量引物为通用引物341F:534R(Hou et al., 2014), 而Anammox菌采用特异性引物Amx808F(5′-ARC YGT AAA CGA TGG GCA CTA A-3′)和Amx1040R (5′-CAG CCA TGC AAC ACC TGT RAT A-3′) (Hou et al., 2014;Meng et al., 2010).实时定量PCR运行程序为三步法:95 ℃预变性5 min;95 ℃变性30 s, 45 ℃退火30 s, 72 ℃延伸30 s, 35个循环;72 ℃终延伸10 min, 最后进行溶解曲线分析.
3 结果与讨论(Results and discussion) 3.1 厌氧氨氧化反应器活性恢复中脱氮性能变化由反应器进出水氮浓度(图 2)和脱氮效率变化(图 3a)可知, 活性恢复中随着进水总氮浓度的阶段性增加, 出水总氮浓度也逐渐增加, 以出水NO3--N浓度增加最为显著, NO2--N次之; 而NH4+-N浓度始终保持在5 mg · L-1以下, 仅在进水氮浓度提高的48 h内, 出水NH4+-N浓度偏高, 但均会迅速趋于稳定.反应器的TN去除率在阶段Ⅰ~Ⅱ逐渐增加, 最高可达91.74%, 在阶段Ⅲ~Ⅴ则逐渐稳定在78.8%左右.而NO2--N去除率相反, 在阶段Ⅰ~Ⅱ稳定于98%左右, 而阶段Ⅲ~Ⅴ则逐渐下降, 阶段Ⅴ去除率平均为83.8%.NH4+-N去除率相对稳定, 始终维持在98%左右.
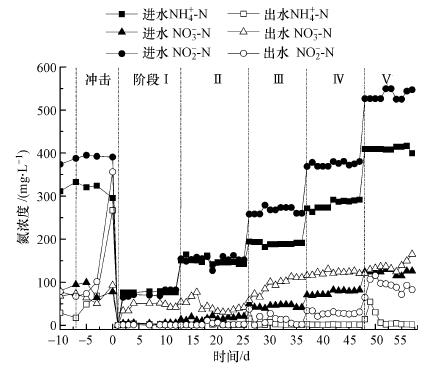 |
| 图 2 活性恢复中反应器进出水氮浓度变化 Fig. 2 Nitrogen variation of influent and effluent during recovery stage of anammox reactor |
由图 3b可知, 活性恢复阶段Ⅰ~Ⅴ中, 氮去除负荷(Nitrogen Removal Rate, NRR)随氮负荷率(Nitrogen Loading Rate, NLR)的增加而逐渐增加, 各阶段NRR分别稳定在0.21、0.64、1.05、1.55和2.21 kg · m-3 · d-1左右, 与Zhang等(2015)报道的重金属铜抑制后Anammox工艺恢复状况相近.因此, 阶段式提高NLR可有效利用菌群的适应性和竞争机制(Sheng et al., 2010), 利于Anammox活性的快速恢复.
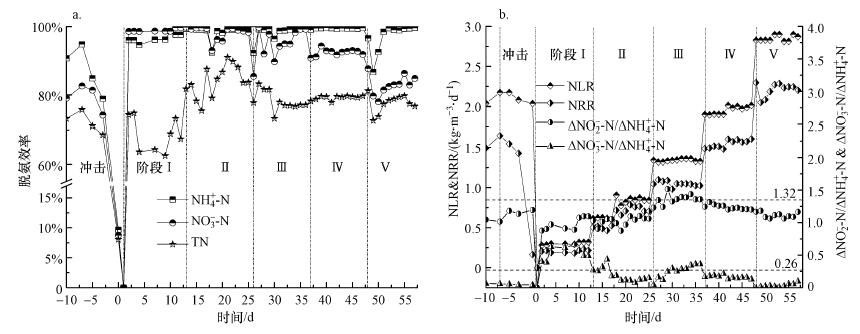 |
| 图 3 厌氧氨氧化反应器中脱氮效率(a)、氮负荷及去除的不同形式氮素比值(b)的变化 Fig. 3 Nitrogen removal performance of the anammox reactor, including nitrogen removal efficiency(a), nitrogen loading rate, ΔNO2--N/ΔNH4+-N and ΔNO3--N /ΔNH4+-N (b) |
由图 2和图 3可知, 在活性恢复阶段(Ⅰ~Ⅳ), 反应器均可在提高NLR后的24 h内快速适应并稳定运行, 截至阶段Ⅳ, 反应器已恢复至受损前的稳定状态.其中, 阶段Ⅱ第18 d时反应器出现较大波动, 使整体脱氮效率呈现较低状态, 且ΔNO3--N/ΔNH4+-N值低于阶段Ⅲ, 可能是因为HRT由12 h缩短至9 h导致的.而在阶段Ⅴ中, NLR提高后反应器需72 h方可渐渐适应, 且NH4+-N和NO2--N去除率分别较阶段Ⅳ降低了2.44%和10.23%, 可能是高浓度的NO2--N对Anammox菌和异养菌有毒害作用, 细胞死亡自溶使反应器内源性COD增加(Tian et al., 2013), 增加了反硝化菌的竞争力.同时, 在进水NO2--N/NH4+-N为1.32的前提下, 尽管阶段Ⅰ~Ⅴ的NH4+-N去除率均高于96%, 但NO2--N去除率和ΔNO3--N/ΔNH4+-N值却逐渐下降, 且出水NO2--N浓度由起初的0.79 mg · L -1渐增至91.00 mg · L-1, 说明虽然有出水回流的稀释作用, 会一定程度上缓解NO2--N对Anammox菌的毒害, 但高浓度NO2--N仍然会抑制Anammox菌活性(Fernández et al., 2012;Kimura et al., 2010;Tang et al., 2010).有研究指出, 当NO2--N浓度超过750 mg L-1时, 90%的Anammox菌发生可逆性失活(Kimura et al., 2010).研究也表明, 瞬时1000 mg · L -1 TN(NH4+-N+NO2--N)的冲击会引起50%Anammox菌失活(Lotti et al., 2012).
3.2 活性恢复阶段厌氧氨氧化菌的EPS组分变化由图 4可知, 反应器活性恢复中EPS含量随NLR提高呈先下降后上升的趋势, 阶段Ⅰ~Ⅴ的EPS含量分别为150.56、33.51、8.42、10.05和10.21 mg · g-1(以VSS计), 各阶段TB-EPS含量均高于LB-EPS含量, 且TB-EPS较LB-EPS对环境敏感度高, 其PN含量均高于PS含量, 这与Jia等(2017)和Pellicernàcher等(2013)的研究结果一致.阶段Ⅰ~Ⅲ中, EPS含量逐渐下降, 阶段Ⅰ中EPS含量远高于阶段Ⅱ和Ⅲ, 其TB-EPS约为LB-EPS的90.25倍, 且TB-PN/PS和LB-PN/PS值分别为21.02和2.21左右, 说明高浓度基质冲击时, 反应器内部分微生物发生了菌体自溶(Tian et al., 2013), 释放出了细胞内部的PN, 使TB-EPS中PN含量剧增, 而PN中荷负电氨基酸较多, 疏水性强(Raszka et al., 2010;Zhang et al., 2007), 利于絮体聚集, 加速了Anammox菌恢复稳定, 同时, TB-EPS紧附于细胞壁上不易脱落(Li et al., 2007;Yang et al., 2009), 导致TB-EPS中PN滞留, 使阶段Ⅰ的TB-EPS含量较高.
 |
| 图 4 活性恢复中反应器内EPS组分变化 Fig. 4 Variation of the EPS composition during recovery stage of anammox reactor |
阶段Ⅰ~Ⅲ, TB-EPS和LB-EPS中PS含量逐渐增加, 易于形成三维网状结构, 利于PN和PS的相互协作及细胞间物质转换和能量传递, 同时, 增加Anammox菌和TB-EPS中EPS修饰酶活性, 使EPS分层更加趋于稳定(Flemming et al., 2010).阶段Ⅳ和Ⅴ, TB-PN/PS和LB-PN/PS值稳定于0.84左右, 此结果与Jia等(2017)报道的Anammox菌稳定时的结果相近, 说明Anammox系统已处于稳态.另外, 阶段Ⅳ和Ⅴ中EPS含量较阶段Ⅲ分别增加了19.36%和21.26%, 是因为TN浓度超过了Anammox菌的合适阈值使其产生一定程度的应激性, 加速了EPS的分泌, 以增强对外界环境变化的耐受性(Hou et al., 2015;Neyens et al., 2004).
3.3 活性恢复阶段Anammox菌的丰度变化从图 5中Anammox菌丰度变化可知, 反应器活性恢复阶段Ⅰ~Ⅴ中细菌总数逐渐上升, 而Anammox菌丰度为7.7×109~2.4×1010 copies · g-1(以VSS计), 介于高梦佳等(2016)和王衫允等(2016)报道的数据之间.Anammox菌的相对丰度与其绝对丰度变化趋势相同, 阶段Ⅰ~Ⅴ分别为7.78%、5.73%、4.14%、12.59%和7.46%.阶段Ⅰ~Ⅲ中, Anammox菌丰度相当, 这说明中断回流1周后Anammox菌数量并没有显著变化, 而其活性受损才是脱氮性能降低的主要原因.在阶段Ⅳ中Anammox菌丰度最高, 为2.4×1010 copies · g-1(以VSS计), 这显示Anammox菌重新适应了反应器的运行条件, 活性得到恢复(Ma et al., 2012;Molin et al., 2003).随后的阶段Ⅴ中, Anammox菌丰度略有下降, 可能与过高的进水NH4+-N和NO2--N浓度的抑制作用有关(Dapena-Mora et al., 2007;Isaka et al., 2007;Raudkivi et al., 2017;Strous et al., 1999;Yang et al., 2011).
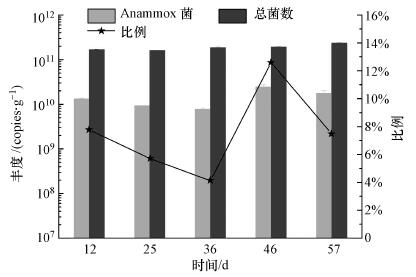 |
| 图 5 活性恢复中反应器内Anammox菌丰度变化 Fig. 5 Changes in abundance of anammox bacteria during recovery stage of anammox reactor |
综合反应器活性恢复过程各阶段的脱氮性能、EPS组分及Anammox菌丰度变化可知, 逐步提高氮负荷, 受损反应器中Anammox菌的活性逐步恢复.TN浓度为700 mg · L-1时, 脱氮效率和Anammox菌丰度较高, 且EPS组分含量适宜.而过高的TN浓度(1000 mg · L-1)条件下, 反应器虽然仍有良好的脱氮效率, 但EPS组分含量及Anammox菌丰度均呈现一定程度恶化(Hou et al., 2015;Lotti et al., 2012), 随着时间延长, 有可能导致其脱氮效率下降.
4 结论(Conclusions)Anammox菌对废水中氮浓度变化敏感.高于700 mg · L-1的TN浓度会导致Anammox菌产生基质抑制, 使Anammox污泥中EPS、TB-PN/PS和LB-PN/PS显著增高.采用阶段式提高负荷有利于Anammox菌的活性恢复, 最终平均氮去除负荷达2.21 kg · m-3 · d-1.TN浓度为700 mg · L-1时反应器运行效果最佳, TN去除率最高为79.74%, 且Anammox菌丰度最高.
Adav S S, Lee D J, Show K Y, et al. 2008. Aerobic granular sludge:Recent advances[J]. Biotechnology Advances, 26(5): 411–423.
DOI:10.1016/j.biotechadv.2008.05.002
|
Badireddy A R, Chellam S, Gassman P L, et al. 2010. Role of extracellular polymeric substances in bioflocculation of activated sludge microorganisms under glucose-controlled conditions[J]. Water Research, 44(15): 4505–4516.
DOI:10.1016/j.watres.2010.06.024
|
Carvajalarroyo J M, Puyol D, Li G, et al. 2015. The role of pH on the resistance of resting-and active anammox bacteria to NO2- inhibition[J]. Biotechnology & Bioengineering, 111(10): 1949–1956.
|
Dapena-Mora A, Campos I F L. 2007. Evaluation of activity and inhibition effects on Anammox process by batch tests based on the nitrogen gas production[J]. Enzyme & Microbial Technology, 40(4): 859–865.
|
Fernández I, Dosta J, Fajardo C, et al. 2012. Short-and long-term effects of ammonium and nitrite on the Anammox process[J]. Journal of Environmental Management, 95(2): 170–174.
|
Flemming H, Wingender J. 2010. The biofilm matrix[J]. Nat Rev Microbiol, 8: 623–633.
DOI:10.1038/nrmicro2415
|
高梦佳, 王淑莹, 王衫允, 等. 2016. 脉冲式流量波动对厌氧氨氧化UASB反应器的影响[J]. 中国环境科学, 2016, 36(8): 2347–2354.
|
国家环境保护总局《水和废水监测分析方法》编委会. 2002. 水和废水监测分析方法(第四版)[M]. 北京: 中国环境科学出版社.
|
Hendrickx T L G, Kampman C, Zeeman G, et al. 2014. High specific activity for anammox bacteria enriched from activated sludge at 10℃[J]. Bioresource Technology, 163: 214–221.
DOI:10.1016/j.biortech.2014.04.025
|
He S, Zhang Y, Niu Q, et al. 2016. Operation stability and recovery performance in an Anammox EGSB reactor after pH shock[J]. Ecological Engineering, 90: 50–56.
DOI:10.1016/j.ecoleng.2016.01.084
|
Hou L, Zheng Y, Liu M, et al. 2014. Anaerobic ammonium oxidation (anammox) bacterial diversity, abundance, and activity in marsh sediments of the Yangtze Estuary[J]. Journal of Geophysical Research Biogeosciences, 118(3): 1237–1246.
|
Hou X, Liu S, Zhang Z. 2015. Role of extracellular polymeric substance in determining the high aggregation ability of anammox sludge[J]. Water Research, 75: 51–62.
DOI:10.1016/j.watres.2015.02.031
|
Isaka K, Sumino T, Tsuneda S. 2007. High nitrogen removal performance at moderately low temperature utilizing anaerobic ammonium oxidation reactions[J]. Journal of Bioscience & Bioengineering, 103(5): 486–490.
|
Jia F, Yang Q, Liu X, et al. 2017. Stratification of extracellular polymeric substances (EPS) for aggregated anammox microorganisms[J]. Environmental Science & Technology, 51(6): 3260–3268.
|
Jin R C, Yang G F, Ma C, et al. 2012a. Influence of effluent recirculation on the performance of Anammox process[J]. Chemical Engineering Journal, 200-202(34): 176–185.
|
Jin R C, Yang G F, Yu J J, et al. 2012b. The inhibition of the Anammox process:A review[J]. Chemical Engineering Journal, 197(29): 67–79.
|
Kimura Y, Isaka K, Kazama F, et al. 2010. Effects of nitrite inhibition on anaerobic ammonium oxidation[J]. Applied Microbiology & Biotechnology, 86(1): 359–365.
|
Li G, Carvajalarroyo J M, Sierraalvarez R, et al. 2017a. Mechanisms and control of NO2- Inhibition of anaerobic ammonium oxidation (Anammox)[J]. Water Environment Research, 89(4): 330–336.
DOI:10.2175/106143017X14839994523064
|
Li J, Zhu W, Dong H, et al. 2017b. Performance and kinetics of ANAMMOX granular sludge with pH shock in a sequencing batch reactor[J]. Biodegradation(5/6): 1–15.
|
Li X Y, Yang S F. 2007. Influence of loosely bound extracellular polymeric substances (EPS) on the flocculation, sedimentation and dewaterability of activated sludge[J]. Water Research, 41(5): 1022–1030.
DOI:10.1016/j.watres.2006.06.037
|
Lotti T, Wr V D S, Kleerebezem R, et al. 2012. The effect of nitrite inhibition on the anammox process[J]. Water Research, 46(8): 2559–2569.
DOI:10.1016/j.watres.2012.02.011
|
Ma C, Jin R C, Yang G F, et al. 2012. Impacts of transient salinity shock loads on Anammox process performance[J]. Bioresource Technology, 112(1): 124–130.
|
Meng L, Hong Y, Klotz M G, et al. 2010. A comparison of primer sets for detecting 16S rRNA and hydrazine oxidoreductase genes of anaerobic ammonium-oxidizing bacteria in marine sediments[J]. Applied Microbiology & Biotechnology, 86(2): 781–790.
|
Molin S, Tolker-Nielsen T. 2003. Gene transfer occurs with enhanced efficiency in biofilms and induces enhanced stabilisation of the biofilm structure[J]. Current Opinion in Biotechnology, 14(3): 255–261.
DOI:10.1016/S0958-1669(03)00036-3
|
Neyens E, Baeyens J, Dewil R, et al. 2004. Advanced sludge treatment affects extracellular polymeric substances to improve activated sludge dewatering[J]. Journal of Hazardous Materials, 106(2/3): 83–92.
|
欧清梅. 2015. 生物膜-qMBR工艺与膜污染缓解机制研究[D]. 哈尔滨: 哈尔滨工业大学
|
Pellicernàcher C, Domingofélez C, Mutlu A G, et al. 2013. Critical assessment of extracellular polymeric substances extraction methods from mixed culture biomass[J]. Water Research, 47(15): 5564–5574.
DOI:10.1016/j.watres.2013.06.026
|
Puyol D, Carvajalarroyo J M, Sierraalvarez R, et al. 2014. Nitrite (not free nitrous acid) is the main inhibitor of the anammox process at common pH conditions[J]. Biotechnology Letters, 36(3): 547–551.
DOI:10.1007/s10529-013-1397-x
|
Raszka A, Chorvatova M, Wanner J. 2010. The role and significance of extracellular polymers in activated sludge.Part Ⅰ:Literature review[J]. CLEAN-Soil, Air, Water, 34(5): 411–424.
|
Raudkivi M, Zekker I, Rikmann E, et al. 2017. Nitrite inhibition and limitation-the effect of nitrite spiking on anammox biofilm, suspended and granular biomass[J]. Water Science & Technology, 75(2): 313–321.
|
Sheng G P, Yu H Q, Li X Y. 2010. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems:a review[J]. Biotechnology Advances, 28(6): 882–894.
DOI:10.1016/j.biotechadv.2010.08.001
|
Strous M. 1999. Key physiology of anaerobic ammonium oxidation[J]. Applied & Environmental Microbiology, 65(7): 3248–3250.
|
Tang C J, Zheng P, Mahmood Q, et al. 2010. Effect of substrate concentration on stability of anammox biofilm reactors[J]. Journal of Central South University of Technology, 17(1): 79–84.
DOI:10.1007/s11771-010-0014-6
|
Tian W D, An K J, Ma C, et al. 2013. Partial nitritation for subsequent Anammox to treat high-ammonium leachate[J]. Environmental Technology, 34(5/8): 1063–1068.
|
王衫允, 贾方旭, 高梦佳, 等. 2016. 不同基因水平的厌氧氨氧化污泥功能微生物特性[J]. 中国给水排水, 2016(13): 96–101.
|
Wang Y, Ma X, Zhou S, et al. 2016. Expression of the nirS, hzsA, and hdh genes in response to nitrite shock and recovery in Candidatus Kuenenia stuttgartiensis[J]. Environmental Science & Technology, 50(13): 6940–6947.
|
Wr V D S, Abma W R, Blommers D, et al. 2007. Startup of reactors for anoxic ammonium oxidation:experiences from the first full-scale anammox reactor in Rotterdam[J]. Water Research, 41(18): 4149–4163.
DOI:10.1016/j.watres.2007.03.044
|
Yang J, Zhang L, Hira D, et al. 2011. High-rate nitrogen removal by the anammox process at ambient temperature[J]. Bioresource Technology, 102(2): 672–676.
DOI:10.1016/j.biortech.2010.08.039
|
Yang S F, Li X Y. 2009. Influences of extracellular polymeric substances (EPS) on the characteristics of activated sludge under non-steady-state conditions[J]. Process Biochemistry, 44(1): 91–96.
DOI:10.1016/j.procbio.2008.09.010
|
Yin C, Meng F, Chen G H. 2015. Spectroscopic characterization of extracellular polymeric substances from a mixed culture dominated by ammonia-oxidizing bacteria[J]. Water Research, 68(68C): 740–749.
|
Yu G H, He P J, Shao L M. 2009. Characteristics of extracellular polymeric substances (EPS) fractions from excess sludges and their effects on bioflocculability[J]. Bioresource Technology, 100(13): 3193–3198.
DOI:10.1016/j.biortech.2009.02.009
|
张俊珂, 曾萍, 宋永会, 等. 2016. 受四环素影响的活性污泥胞内外聚合物特征[J]. 中国环境科学, 2016, 36(3): 751–758.
|
Zhang L, Feng X, Zhu N, et al. 2007. Role of extracellular protein in the formation and stability of aerobic granules[J]. Enzyme & Microbial Technology, 41(5): 551–557.
|
Zhang Q Q, Yang G F, Wang H, et al. 2013. Estimating the recovery of ANAMMOX performance from inhibition by copper (Ⅱ) and oxytetracycline (OTC)[J]. Separation & Purification Technology, 113(29): 90–103.
|
Zhang Z Z, Cheng Y F, Zhou Y H, et al. 2015. A novel strategy for accelerating the recovery of an anammox reactor inhibited by copper(Ⅱ):EDTA washing combined with biostimulation via low-intensity ultrasound[J]. Chemical Engineering Journal, 279: 912–920.
DOI:10.1016/j.cej.2015.05.081
|
Zhu P, Long G, Ni J, et al. 2009. Deposition kinetics of extracellular polymeric substances (EPS) on silica in monovalent and divalent salts[J]. Environmental Science & Technology, 43(15): 5699–5704.
|
 2018, Vol. 38
2018, Vol. 38


