2. 日本国立岐阜大学工学部, 日本岐阜市 501-1193
2. Faculty of Engineering, Gifu University, Gifu 501-1193
城市污泥是污水处理的终端副产物;不仅产量巨大, 而且含有重金属、病原菌和抗性基因等多种有害物质, 致使其处理处置已成为全球性难题(郭广慧等, 2016).相比而言, 蚯蚓堆肥已被证实为一种具有竞争力的可持续的污泥资源化技术(陈学民等, 2010;Su et al., 2016).诸多研究表明:蚯蚓堆肥过程中不仅可以控制污泥中多种环境污染物质(周波等, 2015), 而且可以促使有机物降解转化(Su et al., 2016;Swati et al., 2017;伏小勇等, 2017).并且, 蚯蚓堆肥最终产物蚯蚓粪富含植物性可利用营养元素(有效态氮、磷、钾)及多元化的农业益生菌群, 是一种高效的微生物有机肥(Pathma et al., 2013;Huang et al., 2017).
蚯蚓堆肥系统是一个蚯蚓和微生物协同作用的有机物降解体系(Domínguez et al., 2010).研究表明蚯蚓是有机物降解的主要贡献者(Domínguez et al., 2010).一方面蚯蚓通过自身营养性行为肠道消化, 对有机物起到直接降解的作用(Aira et al., 2008);另一方面蚯蚓的非营养性行为粘液分泌, 排粪、做穴等也会影响微生物的生长繁殖, 间接刺激有机物的降解(Hoang et al., 2016;Aira et al., 2016;Huang et al., 2018).因此, 研究蚯蚓对微生物特征的影响有利于揭示蚯蚓如何加速堆肥体系有机物降解的机理, 而且有助于探明蚯蚓粪微生物肥料的利用价值.一般而言, 微生物的特征包括微生物数量、活性和种群结构(Blagodatskaya et al., 2013).目前虽有学者研究报道了蚯蚓对污泥中微生物数量和活性的影响(徐轶群等, 2010;伏小勇等, 2015), 但对于蚯蚓对污泥堆肥中微生物种群的影响, 相关报道比较鲜见.
因此, 本研究以污泥中的微生物为研究对象, 选用赤子爱胜蚓为堆肥蚓种, 比较接种蚯蚓和无蚯蚓对污泥堆肥过程中微生物特征的变化, 揭示蚯蚓活动对微生物数量、活性和种群的变化的影响, 旨在为蚯蚓堆肥处理污泥提供理论依据.
2 材料和方法(Materials and methods) 2.1 供试材料选用健康的无环带的赤子爱胜蚓(Eisenia fotida)为堆肥蚓种, 体重约为0.3 g, 为本实验室饲养.选用底部开孔的长方形塑料箱(46 cm×17 cm×13 cm)作为堆肥反应器.脱水污泥采自兰州市安宁区七里河污水厂脱泥车间的新鲜污泥, 其物理化学性质见表 1.
| 表 1 污泥的理化性质 Table 1 Physico-chemical properties of sludge used in this study |
取6个蚯蚓堆肥反应器, 每个反应器均放入4 kg新鲜污泥, 投放厚度约为50 mm.为避免污泥堆体缺氧, 把大块新鲜污泥分解成小块后投加.其中3个反应器各接种100条赤子爱胜蚓作为蚯蚓处理组, 另外3个反应器不接种蚯蚓作为对照组.接种蚯蚓数量和污泥投加量参照先前的研究设定(伏小勇等, 2015).所有反应器盖上黑色遮光布避光.每隔3 d喷洒少量水分, 保持湿度在60%~70%左右.实验在室温下进行(18~25 ℃).每20 d取1次样, 实验共进行80 d.所取样品一式3份, 鲜样直接用于酶活性分析;一份在室内自然风干后研磨过80目筛, 放在4 ℃冰箱保存;另一份保存在-20 ℃, 用于DNA相关分析.
2.3 测试方法 2.3.1 腐殖化度评价水溶性有机碳的浓度和结构特征是评价蚯蚓堆肥产物腐熟度的有效手段(Xing et al., 2012;Fernández-Gómez et al., 2015).取0.2 g干样溶于10 mL超纯水, 震荡摇匀后, 过0.45 μm的滤膜, 稀释10倍后作为待测样品储存于4 ℃.一部分用TOC仪(岛津, 日本)测定DOC的浓度;另一部分用荧光分光光度计(岛津, RF-5300PC)测定可溶性有机物的三维荧光结构.所得数据中, A435~480/A300~345被用来作为腐殖质化的评定(Chen et al., 2003;Huang et al., 2017).
2.3.2 脱氢酶活性脱氢酶的测定采用氯化三苯基四氮唑(TTC)比色法(Huang et al., 2013).准确称取0.5 g新鲜样品于5 mL超纯水, 振荡摇匀后, 加1% TTC在37 ℃培养4 h后, 用甲苯萃取红色TPF, 在485 nm下, 用分光光度计测定.
2.3.3 DNA提取和荧光定量PCR所取平行样品混合后作为一个DNA样本, 用DNeasy© PowerSoil© Kit(QIAGEN, 德国)试剂盒提取总DNA, 并用1%的琼脂糖凝胶电泳检测浓度.
采用16S rDNA通用引物Com1 (5′-CAGCAGCCGCGGTAATAC-3′)和Com2 (5′-CCGTCAATTCCTTTGAGTTT-3′)对细菌数量进行定量.25 μL的荧光定量PCR体系为:12.5 μL的荧光染料SYBR ® Green Ⅱ(TAKARA), 10 μmol上下游引物各1 μL, DNA模板2 μL, DNA-free超纯水8.5 μL.PCR反应条件为:95 ℃预变性2 min, 40个循环包括95 ℃变性15 s, 50 ℃退火30 s, 72 ℃延伸30 s.采用10倍连续稀释的E.coli质粒作为标准曲线.用DNA-free超纯水作为阴性对照, 每个样品做3次重复.
2.3.4 PCR和高通量测序采用带有Barcode信息的引物341F (5′-CCTAYGGGRBGCASCAG-3′)和806R (5′-GGACTACNNGGGTATCTAAT-3′)对细菌16S rDNA的V3-V4区进行扩增.高保真酶Phusion ®High-Fidelity PCR Master Mix with GC Buffer(New England Biolabs, 美国)用来进行PCR扩增.所得PCR产物使用2%浓度的琼脂糖凝胶进行电泳检测回收, 并用GeneJET胶回收试剂盒(Thermo Scientific, 美国)纯化.使用Ion Plus Fragment Library Kit 48 rxns建库试剂盒(Thermofisher, 美国)进行文库的构建, 检测合格后, 使用Ion S5TMXL(Thermofisher, 美国)进行上机测序(诺禾致源生物信息科技有限公司, 北京).测序结果用Cutadapt软件对低质量部分剪切后, 序列通过UCHIME Algorithm和数据库Gold database进行比对, 检测嵌合体序列, 最终得到有效的测序数据.
2.4 统计方法使用Statistica10软件对DOC、脱氢酶活性、细菌16S rDNA数量在蚯蚓组和对照组之间随时间的差异性进行Measure repeated ANOVA分析.使用Uparse软件对DNA测序数据进行聚类, 将97%相似性的序列聚类成为OTUs.用Mothur方法对OTUs代表序列进行物种注释, 然后与数据库进行比对.使用Qiime软件计算Observed-species, Chao1, Shannon, Goods-coverage等指数.组间差异显著的物种分析利用R软件做组间T-test检验并作图.本研究统计分析的显著性水平为0.05.
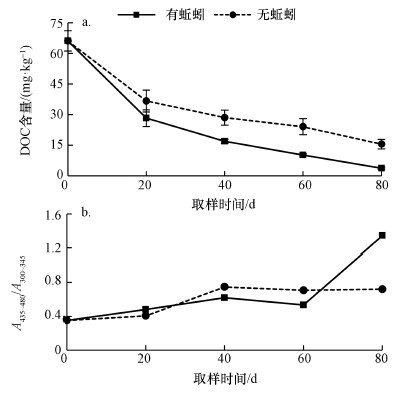
3 结果和讨论(Results and discussion) 3.1 蚯蚓对污泥稳定化的影响污泥稳定化过程是污泥有机质分解转化和腐殖质化的过程.因此, 本研究采用DOC和A435~480/A300~345分别评价有机物降解和腐殖质化的程度(Fernández-Gómez et al., 2015).如图 1a所示, 蚯蚓处理组和对照组的DOC含量在实验过程中均表现出下降的趋势.相比无蚯蚓处理, 蚯蚓组的DOC含量在第20 d呈显著降低趋势(p < 0.05).实验结束后, 蚯蚓处理组和对照组的DOC含量分别为:3.78 mg · kg-1和15.61 mg · kg-1.当蚯蚓堆肥的DOC含量低于4 mg · kg-1时, 则认为其产物较为稳定(Xing et al., 2012).因此, 本研究中蚯蚓组较低的DOC含量说明蚯蚓堆肥产物相对稳定.伏小勇等(2015)研究结果显示蚯蚓在前20 d可以显著提高DOC水平, 激发初期微生物活性, 致使污泥快速稳定.
 |
| 图 1 蚯蚓处理和无蚯蚓污泥中的DOC和腐熟度的变化 Fig. 1 Changes of DOC and humification in vermicomposting and control treatments for recycling sludge |
但对腐殖质化而言, A435~480/A300~345呈相反的趋势.虽然在前60 d, 蚯蚓处理组和对照组并无显著性差异, 但最终产物中蚯蚓处理组的A435~480/A300~345显著高于对照组(p < 0.05), 说明蚯蚓堆肥后期污泥才开始腐殖质化.蚯蚓促进污泥腐殖质化与后期污泥有机质中的类芳香族和蛋白类物质向胡敏酸和富里酸转化有关(Xing et al., 2012;Fernández-Gómez et al., 2015).蚯蚓组较低的DOC含量和较高的腐殖质化水平表明蚯蚓活动可以显著加快有机质的降解, 同时被降解的有机物在后期向腐殖质转化(Xing et al., 2012;杨巍等, 2015), 从而提高了污泥的稳定化程度.
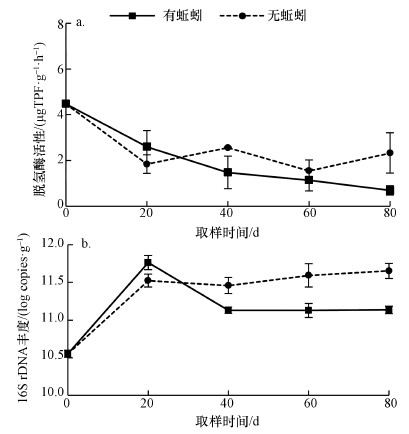
3.2 蚯蚓对微生物数量和活性的影响脱氢酶活性反映微生物对有机物的降解能力, 是堆肥体系中评价微生物活性的重要指标之一.如图 2a所示, 蚯蚓处理组的脱氢酶活性呈平缓降低的趋势, 实验结束后其显著降低了84%.但无蚯蚓处理组的脱氢酶活性前20 d快速降低, 显著低于蚯蚓组, 后续表现出一定的波动.最终, 无蚯蚓组产物的脱氢酶活性比蚯蚓组显著高出70%(p < 0.05).实验初期蚯蚓组和对照组的脱氢酶活性的差异, 可能和初期蚯蚓接种时引入了富含氨基酸类和脂肪酸类物质的粘液, 刺激了初期微生物活性有关(Huang et al., 2018).对照组微生物活性的波动可能和第40 d微生物种群的急剧演变有关.
 |
| 图 2 蚯蚓处理和无蚯蚓污泥中的脱氢酶和细菌数量的变化 Fig. 2 Changes of dehydrogenase activity and bacterial density in vermicomposting and control treatments for recycling sludge |
如图 2b可见, 蚯蚓组的细菌16S rDNA丰度在前20 d增加, 随后呈下降的趋势.而无蚯蚓组的细菌16S rDNA丰度则逐渐增加, 并在第40 d显著高于蚯蚓处理组(p < 0.05).最终产物中, 对照组的细菌16S rDNA含量比蚯蚓组显著高了3.28倍.蚯蚓组初期快速增加的细菌数量可能来源与蚯蚓肠道自身携带的微生物数量(Davidson et al., 2013).另外, 蚯蚓的粘液也会增加堆肥初期的细菌数量(Huang et al., 2018).本研究进一步证实了初期蚯蚓的引入提升了微生物的活性和数量, 从而加速了污泥中有机物的降解.而后期蚯蚓组中较低的有机质和DOC含量导致其微生物活性和数量的显著降低(伏小勇等, 2015).因此, 初期蚯蚓生物量和污泥中可被微生物利用的营养物质显著影响了蚯蚓堆肥体系的微生物活性和数量(Castillo et al., 2013).相比对照组, 蚯蚓处理污泥最终产物的微生物活性和数量均较低, 表明其产物已经稳定(Castillo et al., 2013;Fu et al., 2017).
3.3 蚯蚓对微生物种群的影响如表 2可知, Goods coverage指数全部在0.99以上, 说明本次测序结果可充分反映微生物的真实情况.Chao1和Shannon指数分别反映了微生物种群的丰富度和多样性.如表 2所示, 随着时间的延长, 蚯蚓堆肥和对照组的chao1值均增加, 但在第60 d出现较大波动.从第60 d开始, 接种蚯蚓污泥的Chao1明显高于无蚯蚓污泥, 说明蚯蚓活动增加了堆肥后期的细菌种群丰富度.相比而言, Shannon指数也有类似的趋势, 说明蚯蚓能够显著增加细菌种群多样性.蚯蚓能够增加微生物种群的丰富度和多样性的现象在蚯蚓处理牛粪污泥混合体系(Lv et al., 2015)、果蔬体系(Huang et al., 2013)和蚯蚓生物滤池体系(Xu et al., 2014)等均有发现.蚯蚓肠道是一个稳定的移动微生物库, 富含多种微生物种群, 接种蚯蚓能够将肠道菌排放到堆肥体系中(Drake et al., 2007).另外, 有机物经蚯蚓肠道消化, 被转化成微生物可利用残体排出体外, 进一步刺激多元微生物的生长(Aira et al., 2016).但对于Chao1值在第60 d出现较大波动的原因仍需进一步研究.
| 表 2 蚯蚓处理和无蚯蚓污泥中细菌种群的丰富度和多样性的变化 Table 2 Changes of abundance and diversity of bacterial community in vermicomposting and control treatments for recycling sludge |
蚯蚓堆肥过程细菌菌门的变化见图 3.原污泥样品中细菌种群主要有变形菌门(Proteobacteria)、拟杆菌门(Bacteroidetes)、放线菌门(Actinobacteria)、Saccharibacteria菌门、厚壁菌门(Firmicutes).到第20 d, 污泥中的优势种群为变形菌门、拟杆菌门、厚壁菌门和放线菌门, 丰度占总量的90%以上.对比之下, 此时蚯蚓组和无蚯蚓组并无明显差异.与最初相比, 能够降解大分子有机物如蛋白类、脂肪类、纤维素类的变形菌门、拟杆菌门、厚壁菌门的丰度增加, 可能与初期污泥中大量的微生物残体被降解有关(Chen et al., 2018).到第40 d, 对照组表现出变形菌门丰度略有增加;而接种蚯蚓组中放线菌门和厚壁菌门、Saccharibacteria菌门丰度增加, 但拟杆菌门丰度减少.到第60 d, 对照组微生物菌门以变形菌门(36.6%)和拟杆菌门(39.4%)为主, 蚯蚓组中的优势菌群主要有变形菌门(28.6%)、放线菌门(25.2%)、厚壁菌门(20.3%)和Saccharibacteria菌门(13.00%).此时, 蚯蚓组的细菌种群多样性高于对照组.实验结束时, 无蚯蚓组的变形菌门的丰度持续增加至56.00%, 占绝对优势;其次是拟杆菌门(16.3%)和厚壁菌门(15.4%).较多的变形菌门含量, 这可能和无蚯蚓组的厌氧环境和较高的有机质含量有关.相比而言, 蚯蚓处理污泥产物的优势种群有变形菌(31.3%)、拟杆菌门(27.1%)、放线菌门(21.1%)、Saccharibacteria菌门(4.8%)、绿菌门(3.6%)、厚壁菌门(3.7%)等.
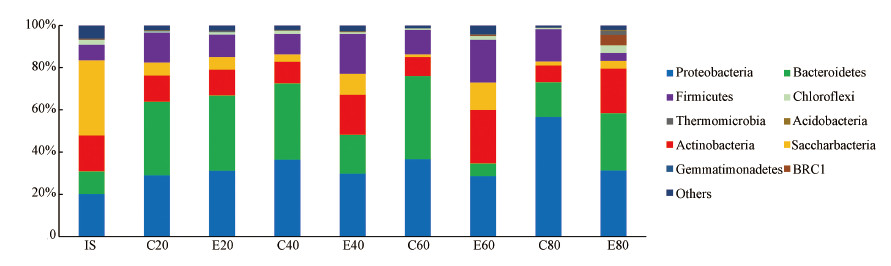 |
| 图 3 蚯蚓处理和无蚯蚓污泥中的细菌种群门水平结构的变化 (注:IS为初始污泥(Initial sludge), C为对照组(Control), E为蚯蚓组(Earthworm);C和E后的20, 40, 60, 80分别为取样时间第20、40、60、80 d.) Fig. 3 Changes of bacterial community structure based on the phylum level in vermicomposting and control treatments for recycling sludge |
从已有的研究来看, Yasir等(2009)发现用蚯蚓堆肥处理牛奶场污泥后产物的优势菌群有变形菌门(47.9%)、拟杆菌门(31.2%)、放线菌门(6.4%);Lv(2015)等而发现污泥和牛粪蚯蚓堆肥后, 蚯蚓粪中变形菌门含量高于50%.这些均与本研究不一致, 这可能和基质中添加了其他拌合物质和不同初始微生物有关(Aira et al., 2016;Knapp et al., 2009;陈浩等, 2012).值得注意的是, 从第40 d开始, 蚯蚓污泥堆肥中就含有较高丰度的放线菌, 包括微球菌属、丙酸杆菌属、双歧杆菌属、放线菌属、棒杆菌属、微单孢菌属、链霉菌属、弗兰克氏菌属等菌属.放线菌是难降解有机物的主要降解者, 也是抗生素的分泌菌种, 是土壤改良的有益菌种, 也是堆肥体系中腐熟度判定的重要微生物菌落之一(Xiao et al., 2011).蚯蚓堆肥体系中高含量的放线菌可能和蚯蚓的排泄物有关(Knapp et al., 2009).Pathma等(2013)对蚯蚓处理羊粪产物检测发现, 仅微球菌属丰度就高达12%.Huang(2016)等研究发现, 蚯蚓蔬菜堆肥产物中放线菌丰度可达到1010 gene · g-1.
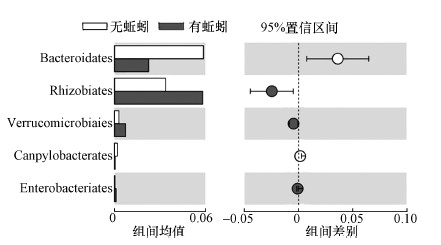
为进一步明确蚯蚓对污泥微生物种群的影响, 本研究对整个实验进程中蚯蚓处理污泥体系和无蚯蚓处理体系的细菌种群(目水平)的差异性进行了比较.如图 4所示, 蚯蚓堆肥系统拟杆菌目(Bacteroidales)和弯曲杆菌目(Campylobacterales)含量显著低于对照组(p < 0.05), 而根瘤菌目(Rhizobiales)、疣微菌目(Verrucomicrobiales)、肠杆菌目(Enterobacteriales)的丰度显著高于对照组(p < 0.05).对照组中较高的拟杆菌含量可能与其高有机质含量有关, 进一步暗示对照产物仍不稳定.蚯蚓堆肥中较低含量的弯曲杆菌表明蚯蚓能够降低原污泥中的病原菌含量(Soobhany et al., 2017).而蚯蚓堆肥系统较高丰度的根瘤菌目、疣微菌目、肠杆菌目与蚯蚓的肠道栖息的微生物群落紧密相关(Knapp et al., 2009;Aira et al., 2016).相关学者发现蚯蚓肠道中含有相当丰度的根瘤菌目(Davidson et al., 2013)、疣微菌目(Knapp et al., 2009;Wüst et al., 2011)、肠杆菌目(Knapp et al., 2009;Wüst et al., 2011)等菌群.但同时, 蚯蚓通过非营养性行为(做穴、粘液、排粪)对基质环境的改造, 也会影响微生物种群的结构(Bernard et al., 2012).蚯蚓污泥堆肥产物中肠杆菌的增加暗示着蚯蚓粪农用仍要注意其潜在的生物风险(Soobhany et al., 2017).放线菌和根瘤菌均属于农业益生菌, 其含量的显著增加表明蚯蚓粪的农用潜力的增加.
 |
| 图 4 蚯蚓处理系统和无蚯蚓处理系统的细菌种群(目水平)的显著性差异 Fig. 4 Significant difference of bacterial community structure based on the order level in vermicomposting and control systems during sludge treatment process |
1) 蚯蚓初期增加了微生物活性和数量, 后期加速了污泥中有机物的腐殖质化.
2) 蚯蚓提升了污泥堆肥产物的微生物种群多样性和丰富度, 变形菌门、拟杆菌门、放线菌门为主要的优势菌群.
3) 蚯蚓增加了蚯蚓粪中放线菌、根瘤菌的含量, 提高了其作为微生物肥料的潜力.
Aira M, Sampedro L, Monroy F, et al. 2008. Detritivorous earthworms directly modify the structure, thus altering the functioning of a microdecomposer food web[J]. Soil Biology & Biochemistry, 40(10): 2511–2516.
|
Aira M, Olcina J, Pérez-Losada M, et al. 2016. Characterization of the bacterial communities of casts from Eisenia andrei, fed with different substrates[J]. Applied Soil Ecology, 98: 103–111.
DOI:10.1016/j.apsoil.2015.10.002
|
Bernard L, Chapuislardy L, Razafimbelo T, et al. 2012. Endogeic earthworms shape bacterial functional communities and affect organic matter mineralization in a tropical soil[J]. ISME Journal, 6(1): 213–222.
DOI:10.1038/ismej.2011.87
|
Blagodatskaya E, Kuzyakov Y. 2013. Active microorganisms in soil:Critical review of estimation criteria and approaches[J]. Soil Biology & Biochemistry, 67: 192–211.
|
陈浩, 赵海涛, 姚振飞, 等. 2012. 饵料对蚯蚓粪细菌群落结构多样性的影响[J]. 农业环境科学学报, 2012, 31(12): 2500–2505.
|
陈学民, 黄魁, 伏小勇, 等. 2010. 2种表居型蚯蚓处理污泥的比较研究[J]. 环境科学, 2010, 31(5): 1274–1279.
|
Castillo J M, Romero E, Nogales R. 2013. Dynamics of microbial communities related to biochemical parameters during vermicomposting and maturation of agroindustrial lignocellulose wastes[J]. Bioresource Technology, 146: 345–354.
DOI:10.1016/j.biortech.2013.07.093
|
Chen W, Westerhoff P, Leenheer J A, et al. 2003. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter[J]. Environmental Science & Technology, 37(24): 5701–5710.
|
Chen Y, Chang S, Chen J, et al. 2018. Characterization of microbial community succession during vermicomposting of medicinal herbal residues[J]. Bioresource Technology, 249: 542–549.
DOI:10.1016/j.biortech.2017.10.021
|
Davidson S K, Powell R, James S. 2013. A global survey of the bacteria within earthworm nephridia[J]. Molecular Phylogenetics & Evolution, 67(1): 188–200.
|
Drake H L, Horn M A. 2007. As the worm turns:the earthworm gut as a transient habitat for soil microbial biomes[J]. Annual Review of Microbiology, 61: 169–189.
DOI:10.1146/annurev.micro.61.080706.093139
|
Domínguez J, Aira M, Gómez-Brandón M. 2010. Vermicomposting: Earthworms Enhance the Work of Microbes[M]//Spriger, Vermicomposting: earthworms enhance the work. 93-114
|
Fernández-Gómez M J, Nogales R, Plante A, et al. 2015. Application of a set of complementary techniques to understand how varying the proportion of two wastes affects humic acids produced by vermicomposting[J]. Waste Management, 35: 81–88.
DOI:10.1016/j.wasman.2014.09.022
|
Fu X, Cui G, Huang K, et al. 2016. Earthworms facilitate the stabilization of pelletized dewatered sludge through shaping microbial biomass and activity and community[J]. Environmental Science & Pollution Research, 23(5): 4522–4530.
|
伏小勇, 崔广宇, 陈学民, 等. 2015. 蚯蚓处理对污泥中微生物碳量及脱氢酶活性的影响[J]. 环境科学学报, 2015, 35(1): 252–256.
|
伏小勇, 张高升, 陈学民, 等. 2017. 城镇污泥蚯蚓堆肥硝化进程及其影响因素[J]. 环境科学学报, 2017, 37(8): 3010–3015.
|
郭广慧, 陈同斌, 雷梅, 等. 2016. 污泥堆肥产物在农业利用中的潜力和问题[J]. 中国给水排水, 2016, 20(32): 34–38.
|
Hoang D T T, Razavi B S, Kuzyakov Y, et al. 2016. Earthworm burrows:Kinetics and spatial distribution of enzymes of C-, N- and P- cycles[J]. Soil Biology & Biochemistry, 99: 94–103.
|
Huang K, Li F, Wei Y, et al. 2013. Changes of bacterial and fungal community compositions during vermicomposting of vegetable wastes by Eisenia foetida[J]. Bioresource Technology, 150(4): 235–241.
|
Huang K, Xia H, Li F, et al. 2016. Optimal growth condition of earthworms and their vermicompost features during recycling of five different fresh fruit and vegetable wastes[J]. Environmental Science & Pollution Research, 23(13): 13569–13575.
|
Huang K, Xia H. 2018. Role of earthworms' mucus in vermicomposting system:Biodegradation tests based on humification and microbial activity[J]. Science of the Total Environment, 610-611: 703–708.
DOI:10.1016/j.scitotenv.2017.08.104
|
Huang K, Xia H, Cui G, et al. 2017. Effects of earthworms on nitrification and ammonia oxidizers in vermicomposting systems for recycling of fruit and vegetable wastes[J]. Science of the Total Environment, 578: 337–345.
DOI:10.1016/j.scitotenv.2016.10.172
|
Knapp B A, Podmirseg S M, Seeber J, et al. 2009. Diet-related composition of the gut microbiota of Lumbricus rubellus, as revealed by a molecular fingerprinting technique and cloning[J]. Soil Biology & Biochemistry, 41(11): 2299–2307.
|
Lv B, Xing M, Yang J, et al. 2015. Pyrosequencing reveals bacterial community differences in composting and vermicomposting on the stabilization of mixed sewage sludge and cattle dung[J]. Applied Microbiology & Biotechnology, 99(24): 10703–10712.
|
Pathma J, Sakthivel N. 2013. Molecular and functional characterization of bacteria isolated from straw and goat manure based vermicompost[J]. Applied Soil Ecology, 70: 33–47.
DOI:10.1016/j.apsoil.2013.03.011
|
Soobhany N, Mohee R, Garg V K. 2017. Inactivation of bacterial pathogenic load in compost against vermicompost of organic solid waste aiming to achieve sanitation goals:A review[J]. Waste Management, 64: 51–62.
DOI:10.1016/j.wasman.2017.03.003
|
Su L L, Leong H L, Ta Y W. 2016. Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation:recent overview, greenhouse gases emissions and economic analysis[J]. Journal of Cleaner Production, 111: 262–278.
DOI:10.1016/j.jclepro.2015.08.083
|
Swati A, Hait S. 2017. Fate and bioavailability of heavy metals during vermicomposting of various organic wastes-A review[J]. Process Safety & Environmental Protection, 109: 30–45.
|
Wüst P K, Horn M A, Drake H L. 2011. Clostridiaceae and Enterobacteriaceae as active fermenters in earthworm gut content[J]. ISME Journal, 5(1): 92–106.
DOI:10.1038/ismej.2010.99
|
徐轶群, 封克, 王子波, 等. 2010. 城市生活污泥蚯蚓处理过程中相关酶活性的动态变化特征[J]. 农业环境科学学报, 2010, 29(5): 995–999.
|
Xing M, Li X, Yang J, et al. 2012. Changes in the chemical characteristics of water-extracted organic matter from vermicomposting of sewage sludge and cow dung[J]. Journal of Hazardous Materials, 205-206: 24–31.
DOI:10.1016/j.jhazmat.2011.11.070
|
Xiao Y, Zeng G M, Yang Z H, et al. 2011. Changes in the actinomycetal communities during continuous thermophilic composting as revealed by denaturing gradient gel electrophoresis and quantitative PCR[J]. Bioresource Technology, 102(2): 1383–1388.
DOI:10.1016/j.biortech.2010.09.034
|
Xu T, Xing M, Yang J, et al. 2014. Tracking the composition and dominant components of the microbial community via polymerase chain reaction-denaturing gradient gel electrophoresis and fluorescence in situ hybridization during vermiconversion for liquid-state excess sludge stabilization[J]. Bioresource Technology, 167(3): 100–107.
|
杨巍, 王东升, 刘满强, 等. 2015. 不同有机物料的蚯蚓堆肥及可溶性有机物的三维荧光光谱特征[J]. 应用生态学报, 2015, 26(10): 3181–3188.
|
Yasir M, Aslam Z, Kim S W, et al. 2009. Bacterial community composition and chitinase gene diversity of vermicompost with antifungal activity[J]. Bioresource Technology, 100(19): 4396–4403.
DOI:10.1016/j.biortech.2009.04.015
|
周波, 唐晶磊, 代金君, 等. 2015. 蚯蚓作用下污泥重金属形态变化及其与化学生物学性质变化的关系[J]. 生态学报, 2015, 19(35): 6269–6279.
|
 2018, Vol. 38
2018, Vol. 38


