药品和个人护理产品(Pharmaceutical and personal care products, PPCPs)是一类主要由分子量为150~1000 DA的物质, 由各种物理化学性质的极性或非极性分子组成的化合物, 是近些年来被广泛关注的新兴污染物(Kim et al., 2009).PPCPs可分为两大类, 即药品和个人护理用品.其中药品的种类繁多, 可包括广泛使用的各类处方药和非处方药, 如抗生素、非甾体类抗炎药、β-受体阻滞剂、脂质调节剂、止痛药、兴奋剂、镇静剂和激素等.个人护理用品的的种类也很多, 如:香料、防晒剂、遮光剂、杀菌消毒剂和洗涤剂等(Bu et al., 2013).药品通常不会完全被人体吸收, 因此未被吸收的部分就会随着排泄进入下水道, 同样个人护理品则会通过人们的日常清洁等途径直接排放.PPCPs能够通过多种途径进入水环境, 包括市政污水、工业废水处理厂直接排放、医院污水、下水道泄漏/下水道溢流、垃圾渗滤液, 以及来自城市或农业区域的地表径流(Launay et al., 2016; Yi et al., 2017).水环境中排放的PPCPs通常可以保留其原始浓度和结构, 或者被物理或生物转化为其他活性(或非活性)化合物(Yang et al., 2017c).
目前, 市政污水处理厂是PPCPs进入水环境的主要途径之一.众所周知, 市政污水处理厂常用的二级生化处理工艺主要是为去除常量有机物、氮和磷而设计的, 以满足最低排放标准, 而对于浓度较低的PPCPs等新兴污染物, 由于其生物降解难, 可能对活性污泥微生物产生相当大的毒性或抑制作用, 导致去除效率较低(Thomaidi et al., 2015).因此二级出水中仍然残留一定浓度(ng·L-1~μg·L-1)的PPCPs, 进入水环境后由于其持久性、毒性和生物活性等可能会对人类的健康乃至整个生态环境存在潜在的风险, 需要进一步的深度处理以消除其不利影响(Yang et al., 2011).
污水处理中常用的深度净化技术有生物净化(Fu et al., 2017; Fu et al., 2019), 纳滤(Yoon et al., 2006)、反渗透(RO)等膜技术(Justo et al., 2015), UV、臭氧等高级氧化工艺(Advanced oxidation processes, AOPs)(Guo et al., 2018)、活性炭吸附(Rostvall et al., 2018)、砂滤(Chan et al., 2018)等.AOPs因其反应条件简单、氧化能力强的特点在污水深度处理、再生水净化和饮用水处理中得到普遍运行(Miklos et al., 2018).而以活性炭作为填料的生物活性炭(Biological activated carbon, BAC)技术因其将活性炭物理吸附作用以及微生物氧化分解协同而具有的高效性和稳定性受到了广泛关注(Ibn Abdul Hamid et al., 2019).而将高级氧化和生物活性炭相结合可以利用两者的优点, AOPs对污染物进行预氧化, 后续BAC进一步处理AOPs阶段的有毒副产物以及未矿化的污染物, 降低整体毒性, 是污水中PPCPs等新兴污染物深度净化和风险削减的有效工艺.本文综述了AOPs-BAC联合工艺深度净化污水中PPCPs的应用及其主要影响因素, 并探讨了该工艺对PPCPs的风险削减能力, 为污水中深度净化工艺的研发及PPCPs生态风险控制提供了参考.
2 污水PPCPs的分布特征与生态风险(Distribution characteristics and ecological risk of PPCPs in wastewater) 2.1 PPCPs的分布特征由于不同地区人口组成、生活习惯和社会经济结构不同, 导致不同污水处理厂进水中PPCPs的类型和浓度存在很大的差异.表 1列举了部分PPCPs在不同地区市政污水的进出水浓度分布和去除效率(Sim et al., 2010; Behera et al., 2011; Luo et al., 2014; Ekpeghere et al., 2018; Rivera-Jaimes et al., 2018).
| 表 1 市政污水中PPCPs分布 Table 1 Distribution of PPCPs in municipal wastewater |
市政污水中PPCPs的调查结果表明大多数区域污水处理厂抗生素进水浓度为 <LOQ~mg·L-1(Kümmerer, 2009; Tran et al., 2018), 其中磺胺类、喹诺酮类抗生素高频检出(Tran et al., 2016; Tran et al., 2018).一般而言, 大多数亚洲国家, 特别是发展中国家的市政污水的抗生素浓度要高于欧洲和北美国家报道的浓度(Duong et al., 2008; Tewari et al., 2013; Yang et al., 2017b);而对于同样研究较多的非甾体抗炎药类, 对乙酰氨基酚在北美国家污水处理厂进水中的浓度则显著高于亚洲和欧洲地区;脂质调节剂类的分布情况类似, 亚洲国家污水处理厂进水中检测到的脂质调节剂吉非罗奇浓度往往比欧洲和北美地区低, 造成这种差异的原因可能与不同地区的肥胖率有关, 北美和欧洲等发达国家的肥胖率显著高于中国、印度等亚洲国家(Tran et al., 2018).卡马西平和舒必利等精神类药物是在欧洲和北美污水处理厂污水中最常检测到的药物.这与该地区人们的生活习惯有关, 有研究表明约1/10的12岁以上的美国人都在服用抗抑郁药, 在中国等非发达国家精神类药物的使用率较低(Gurke et al., 2015; Pratt et al., 2017; Tran et al., 2017).
除了区域分布不均匀之外, 污水中PPCPs的季节性差异也很大.比如在春秋季是流感发生的高峰期, 抗生素等药品的用量会明显上升.而夏季是人们使用沐浴露、避蚊胺、防晒剂等个人护理品的高频时期, 此时污水处理厂相应的进水浓度占比也会相应的增大.
PPCPs在污水处理厂中的去除效率取决于相关的物理化学性质.从表 1可以观察到大多数PPCPs的去除率在20%~100%之间变化, 只有少数低于20%.研究表明, 一般酸性化合物具有中等的去除效率(30%~80%), 例如布洛芬(Wang et al., 2010), 而碱性物质的去除效率更加极端(< 20%或>80%), 例如对乙酰氨基酚在污水处理厂中的去除率大于90%(Tarpani et al., 2018).除此之外, 辛醇水分配系数、生物降解性较高的物质更容易在污泥中被吸附和降解, 例如咖啡因(Li et al., 2018).β受体阻滞剂是用于治疗心血管疾病的常用药物, 通常在污水处理厂的进水和出水中都能检测到, 例如阿替洛尔, 由于它的辛醇水分配系数只有0.16, 吸附性可忽略不计, 不能在污水处理厂得到有效去除(Mohapatra et al., 2016).生物降解/转化是去除非甾体抗炎药的主要机制, 但该类药物在污水处理厂中的去除效率也不高(一般在40%~90%)(Carmona et al., 2014; Fernández-López et al., 2016; Papageorgiou et al., 2016).同时, 还可以观察到一些化合物在污水处理厂中的去除效率是负值, 这是由于它们在污泥中的积累(Li et al., 2011; Gao et al., 2012)以及处理过程中的化学反应产生(Xu et al., 2012).因此, 污水处理厂出水中PPCPs的深度处理及风险削减势在必行.
2.2 PPCPs的生态风险尽管水环境中PPCPs浓度较低, 一些PPCPs在低浓度下仍然具有生物活性, 并且有可能在水生生物中积累, 最终导致水生生态系统可持续性的变化.表 2综述了污水中部分PPCPs的生态风险, 不同级别的PPCPs有可能会导致内分泌干扰、神经毒性、遗传毒性和致变突性等风险.例如, 水环境中ng·L-1级的17α-乙炔雌二醇可以通过分别控制神经传递和解毒系统的乙酰胆碱酯酶(AChE)和谷胱甘肽S-转移酶(GST)的活性来调节水生生物的不同生理学终点的活性(Souza et al., 2013);当鱼长期暴露于脂质调节剂吉非罗奇和非甾体抗炎药双氯酚酸时, GST抑制、脂质过氧化(LPO)和DNA损伤效应明显(Ramirez et al., 2010).
| 表 2 污水中部分PPCPs生态风险 Table 2 Ecological risk of some PPCPs in wastewater |
长时间接触这些化学物质也会对人类健康构成威胁, 即使是在痕量水平下.PPCPs可能会导致许多健康问题, 如过敏, 肺部疾病和癌症等, 类固醇污染物还会导致人类更加肥胖(Jamil, 2019).另一个引起全球关注的问题是部分PPCPs(例如抗生素和抗微生物剂)的存在可导致抗生素抗性基因(ARG)和抗生素抗性细菌(ARB)的传播, 这极大降低了抗生素对人和动物病原体的治疗潜力.这些污染物在饮用水和生活用水中的存在可能会让许多饮用水短缺和废水管理不善的敏感地区面临健康问题和水资源管理等方面的挑战(Sharma et al., 2019).
PPCPs常见的毒性以及风险评估方式主要有遗传毒性、急性毒性、生态风险以及人体健康风险评估等, 不同环境浓度下的各种物质造成的风险也大小不一.例如, Semerjian等(2018)将污水处理厂污水中可量化的10种药物所导致的人类健康风险(风险商, RQs)按从高到低排序为:对乙酰氨基酚>美托洛尔>环丙沙星>红霉素>氧氟沙星>磺胺嘧啶>磺胺甲恶唑>磺胺吡啶>利培酮>磺胺二甲嘧啶.可以看出污水中抗生素类的风险性一般要比其他物质要高, 能在较低浓度(< 10 ng·L-1)下造成环境风险.药物类的研究相较于个人护理品类更多, 特别是在医院污水中, 抗生素类、激素类、非甾体抗炎药等药品在二级出水中的含量很高, 在几十到上万ng·L-1浓度范围内, 能造成较大的急性毒性.饮用水是人类日常最常直接接触到的水体, 其中残留微量的PPCPs(< 1 ng·L-1), 例如咖啡因、磺胺类抗生素等, 仍然会造成人体健康风险.
尽管污水处理系统通常会降低PPCPs毒性, 但是在许多情况下由于PPCPs在污水处理过程的不完全转化, 以及部分吸附态PPCPs的二次释放等导致污水处理厂出水要比进水的毒性更大(Palli et al., 2019).因此, 在衡量不同处理工艺的处理效果时, 掌握了解PPCPs及其代谢产物的浓度-结构-效应关系的相关信息, 对于准确评估PPCPs相关的生态风险是非常必要的.
3 高级氧化联合生物活性炭工艺深度净化污水中PPCPs的工艺原理(Mechanism of AOP-BAC processes for advanced treatment of PPCPs in wastewater)常见的污水深度处理净化工艺有生物净化、絮凝/沉淀-砂滤(CS-SF)、反渗透、颗粒活性炭吸附、紫外(UV)光解和光催化, 以及臭氧化等(Rodriguez-Mozaz et al., 2015; Wang et al., 2018a; Ma et al., 2019).其中生物净化、絮凝/沉淀-砂滤(CS-SF)通常对PPCPs等微污染物的处理效果不够好(Abromaitis et al., 2017), 而反渗透等膜技术成本又太高(Valizadeh et al., 2015).相比之下, 高级氧化工艺(AOPs)因其强氧化能力且对微污染物有很好的去除率(Wardenier et al., 2019)而受到广泛关注, 而由颗粒活性炭(GAC)改性而来的生物活性炭(BAC)技术因为具有活性炭吸附和微生物降解的双重作用而对微污染物有很好的处理效果(Abromaitis et al., 2017), 因此, 两者的联合得到了越来越广泛的应用(Wang et al., 2019).将高级氧化预处理和生物活性炭相结合可以利用两者的优点, 相较于其他工艺具有去除效率高、成本可观、不会或很少残留有毒副产物等优点, 达到深度去除微污染物、削减风险的目的.
3.1 高级氧化工艺净化PPCPs原理高级氧化技术(AOPs)是通过一系列物理化学相互作用产生大量高活性的自由基, 由于自由基的强氧化性, 能够将污水中的PPCPs等有机物矿化为二氧化碳、水及小分子化合物, 从而实现良好的水质(Mehrjouei et al., 2015).其中, 基于羟基自由基(·OH)的芬顿法、光-芬顿法、TiO2光催化法、UV/H2O2法和O3/H2O2法等高级氧化技术去除水中难降解有机物的效率高(Zhang et al., 2016).·OH与PPCPs的反应如公式(1)所示.
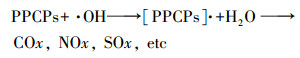
|
(1) |
已有大量研究报道了AOPs在市政污水、饮用水和再生水深度处理中去除PPCPs等微污染物, 减少消毒副产物前体的应用(Antonopoulou et al., 2014; Zoschke et al., 2014; Barbosa et al., 2016).
3.2 生物活性炭工艺净化PPCPs原理生物活性炭工艺(BAC)是通过活性炭吸附作用、微生物降解的协同作用去除PPCPs, 降低其生物风险.如图 1所示, 当PPCPs等有机污染物被吸附到活性炭表面上时, 部分有机分子进入到生物膜基质中, 可生物降解的有机物将会被微生物降解(Scholz et al., 1997), 不可生物降解有机物会在胞外微生物和微孔的共同作用下改性形成可生物降解有机物(Klimenko et al., 2002).当微生物代谢吸附的污染物时, 平衡移动会导致进一步解吸, 直到微生物消耗所有污染物(Vinitnantharat et al., 2001).
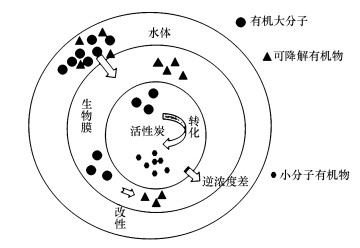 |
| 图 1 BAC作用原理图 Fig. 1 Diagram of the principle of BAC action |
BAC上覆盖的生物膜通常带负电, 因此带有负电和高亲水性的PPCPs不容易被去除, 与此相反, 带有正电和高疏水性的微污染物则更容易在BAC中被降解(Sbardella et al., 2018).Paredes等(2016b)评估了BAC技术处理二级污水的性能, 确定了吸附和生物转化对去除几种PPCPs的贡献.根据在BAC上的去除机制可将PPCPs分为两类:高生物转化和活性炭上的高吸附(例如:布洛芬、萘普生、氟西汀、红霉素、罗红霉素、磺胺甲恶唑、甲氧啶、双酚A、雌酮、雌二醇和乙炔雌二醇, 均属于高疏水性和较易生物降解物质), 被活性炭吸附强, 很少或没有生物转化(例如卡马西平、地西泮, 属于高疏水性物质, 伪一级生物降解动力学常数kbiol < 0.1 L·g-1·d-1, 不易生物转化).另外, 在一些情况下活性炭吸附和微生物降解还会存在竞争机制.例如, Lu等(2019)使用BAC反应器去除双氯酚酸等3种非甾体抗炎药时, 发现在BAC中双氯酚酸、布洛芬、萘普生的最大生物降解率分别为14.04%、25.58%和23.64%, 而在单独的BAC细菌悬浮液测试中, 双氯酚酸、布洛芬、萘普生去除率分别为23.68%、28.89%和25.73%, 远远高于在BAC系统中的生物降解率.结果表明, BAC对药物的去除不仅仅是活性炭-细菌悬浮系统中生物降解和活性炭吸附的简单累积效应, 可能存在竞争机制.
3.3 高级氧化联合生物活性炭工艺净化PPCPs原理尽管高级氧化过程在大多数情况下表现出良好的处理性能, 但有机污染物不会被完全矿化, 而是被部分氧化成转化产物(Transformation product, TPs), 转化产物可能具有一定程度的毒性, 并且增加了处理水体的复杂性(Gerrity et al., 2011).虽然TPs浓度低, 但可能会危害人类和生物体健康, 也有可能损害到生态系统(Prasse et al., 2015; Wang et al., 2017b);在地表水或污水处理厂出水中产生的有毒TPs可能受到基质(例如溶解性有机质)的影响, 导致更复杂的情况, 很有可能造成更高的风险(Borova et al., 2014).例如, Gao等(2018)研究了UV/Na2S2O8降解3种非甾体抗炎药的效率以及它们的转化产物对鲤鱼(C.carpio)的毒性, 结果表明, 非甾体抗炎药的矿化率仅为28%, 且非甾体抗炎药降解产物可能比非甾体抗炎药自身对鲤鱼具有更严重的毒性作用.Wang等(2018b)对精神类药物奥卡西平的臭氧化产物潜在毒性进行了研究, 评估结果表明, 大多数已鉴定的副产物可能比奥卡西平本身毒性要更大.Wang等(2018c)整理近些年来的相关研究发现超过80%的有机污染物在UV-AOPs处理中都观察到了有毒转化产物.因此, AOPs产生的有害转化产物需要进一步处理.相比较其他工艺而言, 采用BAC工艺进一步处理的风险削减效果比较好.同时, 由于BAC的生物处理过程存在一些局限性, 例如, 它主要转化的是易生物降解的化合物, 并且此工艺对其进水温度和水质的变化适应性不是很好.特别是, 针对于转化生物持久性的污染物, 需要通过AOPs预处理将其转化为易降解微污染物, 再使用生物过程吸附降解将其进一步矿化.
因此联合工艺将两者结合, 利用高级氧化工艺将不易被活性炭吸附降解的有机物分解为容易被生物活性炭吸附降解的有机物, 进一步采用BAC工艺降低PPCPs及其代谢产物的浓度, 削减其生态风险, 通过两者的协同作用有效提高AOPs-BAC强化系统对PPCPs的去除效果, 降低有害TPs带来的风险.AOPs-BAC联用技术具有较好的适用性, 而且工艺比较成熟、操作和运行也较为简单稳定, 在工程应用中可以作为较理想的污水深度处理技术(Li et al., 2007; Chen et al., 2017; Yang et al., 2017a).
4 高级氧化联合生物活性炭工艺深度净化污水中PPCPs的应用研究(Advanced treatment of PPCPs in wastewater by AOPs-BAC processes) 4.1 AOPs-BAC联合工艺的应用AOPs-BAC工艺中最常用的联合方式是UV/H2O2-BAC和O3-BAC, 不同的联合工艺对PPCPs的去除效果各有不同.
4.1.1 UV/H2O2-BAC联合工艺UV/H2O2主要通过H2O2光解产生的羟基自由基(·OH)作用.据报道, UV/H2O2可有效降解许多PPCPs, 如双酚A、咖啡因、卡马西平、氯霉素、双氯酚酸、布洛芬、萘普生、美托洛尔、磺胺甲恶唑和甲氧苄啶, 反应速率常数(k)高达109~1010 L·mol-1·s-1(Wols et al., 2012).
UV/H2O2-BAC联合工艺对微污染物具有较好的处理效果, 但是需要较高的H2O2投加量及较长的停留时间, 运行成本较高, 近年来该技术在国内外深度净化处理中的实际应用尚且不广泛.Metz等(2011)采用中试条件下UV/H2O2-BAC降解二级出水中的阿特拉津, 发现200~500 mJ·cm-2中压反应器条件下, 大多数研究季节中阿特拉津的降解率能够达到75%~85%.Li等(2017)研究中试条件下UV/H2O2-BAC深度处理对进水浓度分别是(140±71)、(790±610)、(170±160)μg·L-1的卡马西平、吉非罗奇、磺胺甲噁唑的去除研究发现, 其出水中对应的浓度分别是小于10、26、23 μg·L-1左右的极低水平, 除了磺胺甲噁唑, 去除率基本上都高于90%.
此外, 由于H2O2对UV吸收率很低, 因此通常需要过量的H2O2剂量(Lee et al., 2016), 残留的H2O2对后续产生的影响, 不同研究者的结论不一致.Ksibi(2006)认为H2O2分解产生的O2提高了好氧微生物的生物活性.而Wang等(2017a)的研究则认为低浓度的H2O2可能不会影响微生物活性, 高浓度的H2O2对微生物活性产生负面影响.残留的H2O2对BAC中微生物的具体影响还有待进一步的研究.
4.1.2 O3-BAC联合工艺与UV/H2O2相比, O3的氧化能力更强, 而且不会产生卤代化合物, O3可以破坏水中有机物的结构, 但是也有研究表明, O3会和水中含有较高浓度的溴离子发生反应生成溴酸盐, 而溴酸盐具有较强的毒性, 虽然化学氧化剂对水中有机污染物的去除具有较好的效果, 但是会形成有毒副产物使其推广应用受到局限(Soltermann et al., 2017; Yang et al., 2019).而将O3和BAC工艺联用, 就可以扬长避短, 能更加有效的去除水中有机物质, 会对PPCPs等有机污染物以及消毒副产物前驱物有较好的去除效果(Lee et al., 2012).
O3-BAC在市政污水深度处理中的应用比UV/H2O2-BAC广泛.Sun等(2018)报道, O3-BAC能将二级出水中的大部分PPCPs浓度降低至低于检测限的0.01 μg·L-1之间.Knopp等(2016)研究臭氧加不同生物后处理时, 臭氧阶段对二级出水中浓度为(1.4±0.3)、(9.2±5.6) μg·L-1的卡马西平和碘普罗胺的去除率分别为89%和67%, 而BAC阶段能将碘普罗胺、卡马西平进一步消除至0.05 μg·L-1和0.03 μg·L-1.刘成等(2019)研究太湖流域某典型城市水厂中O3-BAC对阿特拉津等微污染物的去除发现BAC中的生物降解过程对阿特拉津、2-MIB这两类物质的去除效果相对有限, 而吸附作用是生物活性炭去除这两类物质的主要途径, 最高去除率能达到60%以上.安娜等(2018)的研究中, 经O3-BAC工艺处理后的出水中, 有11种PPCPs降至检出限以下, 约占总种类的65%;对氨糖美辛和泰妙菌素的去除率可达95%以上;对卡巴克络、咖啡因、磺胺甲恶唑等PPCPs的去除率最小, 约为7%.
同样残余的O3会对BAC的微生物性能产生影响.Lohwacharin等(2015)研究发现当残余臭氧浓度约为0.88 mg·L-1时, 会影响到BAC床顶部的微生物浓度, 进而减少生物质.除此之外, 残留的O3可以与BAC表面相互作用并增强O3分解成羟基自由基(Xing et al., 2014), 而过量的羟基自由基会对微生物生长有损伤作用.但也有研究发现投加少量的O3对炭层中的生物量并没有显著的影响, 例如陈国强等(2017)进行O3-BAC中试研究发现O3投加与否, O3-BAC出水异养菌数量始终维持在104 CFU·mL-1左右.
4.2 联合工艺去除PPCPs主要影响因素研究 4.2.1 氧化剂剂量氧化剂剂量是影响联合工艺中PPCPs的重要因素, 充足的氧化剂有利于微污染物在AOPs阶段的深度矿化, 但是氧化途径也是影响微污染物的降解效果的关键因素.比如Buffle等(2006)介绍O3与微污染物的反应有两种氧化机制:一种是直接反应, O3分子直接氧化微污染物;另一种是间接反应, 微污染物通过与O3产生的羟基自由基反应, 但由于与浓度比值[O3/(·OH)]通常大于107~109范围内, 因此一般是直接反应起主导作用.Reungoat等(2012)对O3-BAC联合工艺中的O3氧化作用进行研究发现, 分子直接反应速率很高(>104 L·mol-1·s-1)的PPCPs, 比如双氯芬酸、磺胺甲恶唑、甲氧苄啶、普萘洛尔、萘普生、卡马西平、罗红霉素、红霉素等即使在低臭氧剂量下(< 0.5 mg·mg-1 DOC)也能够快速被氧化去除, 低直接反应速率的化合物需要暴露于更高的臭氧剂量以允许其有效氧化.
4.2.2 BAC空床接触时间单位体积颗粒活性炭填料在单位时间内的处理水量, 称为空床接触时间EBCT, 一般以min表示.这是在BAC滤池设计阶段应该考虑的重要因素, 也是影响PPCPs等微污染物降解的主要因素.通过增加床体积或降低通过反应器的流速可以实现更长的EBCT.DOC的去除能够随着EBCT的增加而增加(Gernjak, 2012).Paredes等(2016a)将BAC反应器在25 ℃的恒定温度和不同的EBCT下操作降解PPCPs等微污染物, 研究结果表明降低EBCT具有两个相反的效果:一个阳性(由于流速增加而导致更高的生物活性)和一个阴性(生物转化的时间更短).对于生物降解缓慢的化合物(kbiol < 1 L·g-1·d-1), 例如甲氧苄啶, 磺胺甲恶唑和氟西汀, 消除受到动力学限制, 因此, EBCT的去除效率降低.相反, 对于中等和快速可生物降解的化合物(双酚A、罗红霉素、红霉素、萘普生、17β雌二醇、布洛芬、17α炔雌醇和17β雌二醇), 去除率在较低的EBCT下得到改善, 说明高生物活性相对于接触时间对其更为重要.Tran等(2013)也表示PPCPs等微污染物的生物降解归因于共代谢和/或代谢活性的微生物, 缩小EBCT、增大流速有利于提升生物活性, 对易生物降解微污染物的生物降解具有促进作用.
4.3 联合工艺对风险削减情况探索有效的深度处理方法以降低二级出水的生态风险是一个迫切需要解决的问题.AOPs-BAC联合工艺能够显示出较强的风险削减能力, 可以分为以下几种机制:①AOPs与BAC联合降低毒性:AOPs矿化一部分微污染物, BAC吸附降解高级氧化过程未降解的微污染物, 进而降低毒性.②AOPs未完全矿化微污染物, 生成氧化副产物, BAC进一步降解副产物以削减风险;例如, Chen等(2017)对O3-BAC联合工艺的研究表明臭氧处理可以显著降低雌激素化合物诱导的雌激素活性, 能够将其降到极低水平(雌二醇诱导的β-半乳糖苷酶活性的抑制为(0.01±0.03) μg·L-1, 接近检测限), 在此基础上, 对于臭氧难降解微污染物, 例如咖啡因和布洛芬, BAC对其有进一步的降解去除.同样地, Reungoat等(2012)进行生物发光抑制试验对O3-BAC工艺的风险削减性能评估发现, 整体工艺实现了基线-TEQ(虚拟基线毒物的浓度)生物量的75%±9%和60%±20%的去除.Lu等(2013)通过Microtox测定法评估UV/H2O2-BAC联合处理后的水样发现没有检测到毒性.Rozas等(2017)将O3和粉末活性炭(PAC)混合在一起作为联合工艺, 并采用大型蚤Daphnia magna的毒性试验对比评估了O3和O3-PAC联合降解毒性发现, 与O3-PAC系统相比, 单独使用O3的急性毒性降低得更慢.例如, 在反应15 min后, 分别用O3和O3-PAC系统将急性毒性分别降低45%和80%.Knopp等(2016)探究BAC对O3阶段微污染物转化产物去除结果表明, 曲马多在O3氧化期间5%被转化为曲马多-N-氧化物, 而在后续BAC反应器的流出物中未检测到曲马多-N-氧化物.Bollmann等(2016)在同一实验装置中研究了N-氧化物在3种非典型抗精神病药物氨磺必利, 舒必利和拉莫三嗪中的形成和行为, 与产生的曲马多-N-氧化物类似, 在O3氧化过程中形成了舒必利, 拉莫三嗪-N-氧化物, 并且都能够通过BAC除去.
不过联合工艺的风险评估相关研究仍然太少, 需要更多的补充研究.另外污水中毒性风险诱导的化学物质到目前为止很少报道.污水的毒性可能是由具有特定官能团的化学物质引起的, 微污染物和毒性之间有良好的相关性(Li et al., 2014), 这些都有待进一步的研究.
5 结论(Conclusions)1) PPCPs在不同地区污水处理厂的分布和去除效率存在很大的差异, 其分布特征主要受地区人民生活习惯影响, 而去除效率主要取决于其自身辛醇水分配系数、生物降解性等物理化学性质.PPCPs长期在水生生物体内累积会带来急性毒性、遗传毒性等一系列风险, 最终会危害到人类本身.因此需要在进行风险评估的基础上加强对PPCPs的深度强化去除.
2) BAC深度处理工艺能够通过吸附和微生物降解作用有效去除PPCPs等微污染物, 并且处理的效率高于传统水处理工艺.而采用不同的AOPs与BAC结合能够利用两者的优点, 通过预氧化和后续生物吸附作用进一步强化PPCPs的去除, 削减风险.
3) 联合工艺对PPCPs的去除效果主要受氧化剂剂量、空床接触时间两大因素影响, 在一般情况下, 充足的氧化剂有利于PPCPs的氧化降解, 但是过量的氧化剂残留有可能影响到后续BAC的微生物性能.而空床接触时间对PPCPs去除的影响取决于活性炭吸附和BAC上的微生物活性变化.因此在设计AOPs-BAC工艺时, 考虑相应的运行成本之外, 也需要结合去除效率和相关的水力容量限制选择改进工艺参数.除此之外, 当AOPs未能完全矿化PPCPs时, BAC能够吸附降解AOPs过程中形成的有毒副产物和未能矿化的微污染物, 所以AOPs-BAC联合工艺能够显示出较强的风险削减能力.
4) 污水中污染物成分复杂, 以前的研究更为关注对单个污染物的降解, 而对二元或者多元微污染物造成的生态风险、健康风险在处理工艺中的衍变、削减少有关注.单一AOPs和BAC的研究被广泛报道, 但很少有比较研究联合工艺在生物毒性、风险削减降低中如何发挥作用.而且, 微污染物的去除也并不能完全代表污水处理的最终目的, 工艺处理后仍然存在有大量有机物和副产物, 未来还应该对PPCPs等微污染物的矿化或从流出物中识别代谢副产物进行进一步的研究.
Abromaitis V, Racys V, van der Marel P, et al. 2017. Effect of shear stress and carbon surface roughness on bioregeneration and performance of suspended versus attached biomass in metoprolol-loaded biological activated carbon systems[J]. Chemical Engineering Journal, 317(Complete): 503–511.
|
Afonso-Olivares C, Sosa-Ferrera Z, Santana-Rodríguez J J. 2017. Occurrence and environmental impact of pharmaceutical residues from conventional and natural wastewater treatment plants in Gran Canaria (Spain)[J]. Science of the Total Environment, 599-600: 934–943.
DOI:10.1016/j.scitotenv.2017.05.058
|
Antonopoulou M, Evgenidou E, Lambropoulou D, et al. 2014. A review on advanced oxidation processes for the removal of taste and odor compounds from aqueous media[J]. Water Research, 53(8): 215–234.
|
Anumol T, Vijayanandan A, Park M, et al. 2016. Occurrence and fate of emerging trace organic chemicals in wastewater plants in Chennai, India[J]. Environment International, 92-93: 33–42.
DOI:10.1016/j.envint.2016.03.022
|
Archer E, Petrie B, Kasprzyk-Hordern B, et al. 2017. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters[J]. Chemosphere, 174: 437–446.
DOI:10.1016/j.chemosphere.2017.01.101
|
Azzouz A, Ballesteros E. 2013. Influence of seasonal climate differences on the pharmaceutical, hormone and personal care product removal efficiency of a drinking water treatment plant[J]. Chemosphere, 93(9): 2046–2054.
DOI:10.1016/j.chemosphere.2013.07.037
|
安娜, 张德明, 刘嘉琪, 等. 2018. 臭氧生物活性炭工艺净水效果综述[J]. 净水技术, 2018, 37(7): 18–25.
|
Barbosa M, Moreira N F F, Ribeiro A R, et al. 2016. Occurrence and removal of organic micropollutants:an overview of the watch list of EU Decision 2015/495[J]. Water Research, 94: 257–279.
DOI:10.1016/j.watres.2016.02.047
|
Behera S K, Kim H W, Oh J E, et al. 2011. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea[J]. Science of the Total Environment, 409(20): 4351–4360.
DOI:10.1016/j.scitotenv.2011.07.015
|
Benotti M J, Trenholm R A, Vanderford B J, et al. 2009. Pharmaceuticals and endocrine disrupting compounds in U. S. drinking water[J]. Environmental Science & Technology, 43(3): 597–603.
|
Boleda M R, Galceran M T, Ventura F. 2011. Behavior of pharmaceuticals and drugs of abuse in a drinking water treatment plant (DWTP) using combined conventional and ultrafiltration and reverse osmosis (UF/RO) treatments[J]. Environmental Pollution, 159(6): 1584–1591.
DOI:10.1016/j.envpol.2011.02.051
|
Bollmann A F, Seitz W, Prasse C, et al. 2016. Occurrence and fate of amisulpride, sulpiride, and lamotrigine in municipal wastewater treatment plants with biological treatment and ozonation[J]. Journal of Hazardous Materials, 320: 204–215.
DOI:10.1016/j.jhazmat.2016.08.022
|
Borova V L, Maragou N C, Gago-Ferrero P, et al. 2014. Highly sensitive determination of 68 psychoactive pharmaceuticals, illicit drugs, and related human metabolites in wastewater by liquid chromatography-tandem mass spectrometry[J]. Analytical and Bioanalytical Chemistry, 406(17): 4273–4285.
DOI:10.1007/s00216-014-7819-3
|
Bu Q, Wang B, Huang J, et al. 2013. Pharmaceuticals and personal care products in the aquatic environment in China:A review[J]. Journal of Hazardous Materials, 262: 189–211.
DOI:10.1016/j.jhazmat.2013.08.040
|
Buffle M O, Schumacher J, Salhi E, et al. 2006. Measurement of the initial phase of ozone decomposition in water and wastewater by means of a continuous quench-flow system:Application to disinfection and pharmaceutical oxidation[J]. Water Research, 40(9): 1884–1894.
DOI:10.1016/j.watres.2006.02.026
|
陈国强, 王剑, 张正德, 等. 2017. 臭氧/生物活性炭工艺的微生物泄漏控制研究[J]. 中国给水排水, 2017, 33(15): 36–41.
|
Carmona E, Andreu V, Picó Y. 2014. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin:From waste to drinking water[J]. Science of the Total Environment, 484: 53–63.
DOI:10.1016/j.scitotenv.2014.02.085
|
Chan S, Pullerits K, Riechelmann J, et al. 2018. Monitoring biofilm function in new and matured full-scale slow sand filters using flow cytometric histogram image comparison (CHIC)[J]. Water Research, 138: 27–36.
DOI:10.1016/j.watres.2018.03.032
|
Chang H, Wan Y, Wu S, et al. 2011. Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters:Comparison to estrogens[J]. Water Research, 45(2): 732–740.
DOI:10.1016/j.watres.2010.08.046
|
Chen Z, Li M, Wen Q. 2017. Comprehensive evaluation of three sets of advanced wastewater treatment trains for treating secondary effluent:Organic micro-pollutants and bio-toxicity[J]. Chemosphere, 189: 426–434.
DOI:10.1016/j.chemosphere.2017.09.092
|
Duong H A, Pham N H, Nguyen H T, et al. 2008. Occurrence, fate and antibiotic resistance of fluoroquinolone antibacterials in hospital wastewaters in Hanoi, Vietnam[J]. Chemosphere, 72(6): 968–973.
DOI:10.1016/j.chemosphere.2008.03.009
|
Ekpeghere K I, Sim W J, Lee H J, et al. 2018. Occurrence and distribution of carbamazepine, nicotine, estrogenic compounds, and their transformation products in wastewater from various treatment plants and the aquatic environment[J]. Science of the Total Environment, 640-641: 1015–1023.
DOI:10.1016/j.scitotenv.2018.05.218
|
Fernández-López C, Guillén-Navarro J M, Padilla J J, et al. 2016. Comparison of the removal efficiencies of selected pharmaceuticals in wastewater treatment plants in the region of Murcia, Spain[J]. Ecological Engineering, 95(Complete): 811–816.
|
Frédéric O, Yves P. 2014. Pharmaceuticals in hospital wastewater:Their ecotoxicity and contribution to the environmental hazard of the effluent[J]. Chemosphere, 115: 31–39.
DOI:10.1016/j.chemosphere.2014.01.016
|
Fu J, Lee W N, Coleman C, et al. 2019. Removal of pharmaceuticals and personal care products by two-stage biofiltration for drinking water treatment[J]. Science of the Total Environment, 664: 240–248.
DOI:10.1016/j.scitotenv.2019.02.026
|
Fu J, Lee W N, Coleman C, et al. 2017. Pilot investigation of two-stage biofiltration for removal of natural organic matter in drinking water treatment[J]. Chemosphere, 166: 311–322.
DOI:10.1016/j.chemosphere.2016.09.101
|
Gao P, Ding Y, Li H, et al. 2012. Occurrence of pharmaceuticals in a municipal wastewater treatment plant:Mass balance and removal processes[J]. Chemosphere, 88(1): 17–24.
DOI:10.1016/j.chemosphere.2012.02.017
|
Gao X S, Geng J J, Du Y R, et al. 2018. Comparative study of the toxicity between three non-steroidal anti-inflammatory drugs and their UV/Na2S2O8 degradation products on Cyprinus carpio[J]. Scientific Reports, 8: 11.
DOI:10.1038/s41598-017-18324-8
|
García-Galán M J, González Blanco S, López Roldán R, et al. 2012. Ecotoxicity evaluation and removal of sulfonamides and their acetylated metabolites during conventional wastewater treatment[J]. Science of the Total Environment, 437: 403–412.
DOI:10.1016/j.scitotenv.2012.08.038
|
Gernjak M R J R W. 2012. Organic Micropollutant Removal by Biological Activated Carbon Filtration: A Review[R]. Urban Water Security Research Alliance Technical Report No. 53.
http://www.urbanwateralliance.org.au/publications/UWSRA-tr53.pdf |
Gerrity D, Trenholm R A, Snyder S A J W R. 2011. Temporal variability of pharmaceuticals and illicit drugs in wastewater and the effects of a major sporting event[J]. Water Research, 45(17): 5399–5411.
DOI:10.1016/j.watres.2011.07.020
|
Golovko O, Šauer P, Fedorova G, et al. 2018. Determination of progestogens in surface and waste water using SPE extraction and LC-APCI/APPI-HRPS[J]. Science of the Total Environment, 621: 1066–1073.
DOI:10.1016/j.scitotenv.2017.10.120
|
Guo K, Wu Z, Yan S, et al. 2018. Comparison of the UV/chlorine and UV/H2O2 processes in the degradation of PPCPs in simulated drinking water and wastewater:Kinetics, radical mechanism and energy requirements[J]. Water Research, 147: 184–194.
DOI:10.1016/j.watres.2018.08.048
|
Gurke R, Rößler M, Marx C, et al. 2015. Occurrence and removal of frequently prescribed pharmaceuticals and corresponding metabolites in wastewater of a sewage treatment plant[J]. Science of the Total Environment, 532: 762–770.
DOI:10.1016/j.scitotenv.2015.06.067
|
Ibn Abdul Hamid K, Sanciolo P, Gray S, et al. 2019. Comparison of the effects of ozone, biological activated carbon (BAC) filtration and combined ozone-BAC pre-treatments on the microfiltration of secondary effluent[J]. Separation and Purification Technology, 215: 308–316.
DOI:10.1016/j.seppur.2019.01.005
|
Jamil K. 2019. Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology[M]. 115-128
|
Justo A, González O, Sans C, et al. 2015. BAC filtration to mitigate micropollutants and EfOM content in reclamation reverse osmosis brines[J]. Chemical Engineering Journal, 279: 589–596.
DOI:10.1016/j.cej.2015.05.018
|
Kim I, Tanaka H. 2009. Photodegradation characteristics of PPCPs in water with UV treatment[J]. Environment International, 35(5): 793–802.
DOI:10.1016/j.envint.2009.01.003
|
Klimenko N, Winther-Nielsen M, Smolin S, et al. 2002. Role of the physico-chemical factors in the purification process of water from surface-active matter by biosorption[J]. Water Research, 36(20): 5132–5140.
DOI:10.1016/S0043-1354(02)00278-6
|
Knopp G, Prasse C, Ternes T A, et al. 2016. Elimination of micropollutants and transformation products from a wastewater treatment plant effluent through pilot scale ozonation followed by various activated carbon and biological filters[J]. Water Research, 100: 580–592.
DOI:10.1016/j.watres.2016.04.069
|
Kosma C I, Lambropoulou D A, Albanis T A. 2014. Investigation of PPCPs in wastewater treatment plants in Greece:Occurrence, removal and environmental risk assessment[J]. Science of The Total Environment, 466-467: 421–438.
DOI:10.1016/j.scitotenv.2013.07.044
|
Krzeminski P, Schwermer C, Wennberg A, et al. 2017. Occurrence of UV filters, fragrances and organophosphate flame retardants in municipal WWTP effluents and their removal during membrane post-treatment[J]. Journal of Hazardous Materials, 323: 166–176.
DOI:10.1016/j.jhazmat.2016.08.001
|
Ksibi M. 2006. Chemical oxidation with hydrogen peroxide for domestic wastewater treatment[J]. Chemical Engineering Journal, 119(2): 161–165.
|
Kümmerer K. 2009. Antibiotics in the aquatic environment:A review-Part Ⅰ[J]. Chemosphere, 75(4): 417–434.
DOI:10.1016/j.chemosphere.2008.11.086
|
Launay M A, Dittmer U, Steinmetz H. 2016. Organic micropollutants discharged by combined sewer overflows-Characterisation of pollutant sources and stormwater-related processes[J]. Water Research, 104: 82–92.
DOI:10.1016/j.watres.2016.07.068
|
Lee C O, Howe K J, Thomson B M. 2012. Ozone and biofiltration as an alternative to reverse osmosis for removing PPCPs and micropollutants from treated wastewater[J]. Water Research, 46(4): 1005–1014.
DOI:10.1016/j.watres.2011.11.069
|
Lee Y, Gerrity D, Lee M, et al. 2016. Organic contaminant abatement in reclaimed water by UV/H2O2 and a combined process consisting of O3/H2O2 followed by UV/H2O2:Prediction of abatement efficiency, energy consumption, and byproduct formation[J]. Environmental Science & Technology, 50(7): 3809–3819.
|
Li B, Zhang T. 2011. Mass flows and removal of antibiotics in two municipal wastewater treatment plants[J]. Chemosphere, 83(9): 1284–1289.
DOI:10.1016/j.chemosphere.2011.03.002
|
Li D, Stanford B, Dickenson E, et al. 2017. Effect of advanced oxidation on N-nitrosodimethylamine (NDMA) formation and microbial ecology during pilot-scale biological activated carbon filtration[J]. Water Research, 113: 160–170.
DOI:10.1016/j.watres.2017.02.004
|
Li L, Zhu W, Zhang P, et al. 2007. UV/O3-BAC process for removing organic pollutants in secondary effluents[J]. Desalination, 207(1): 114–124.
|
Li M, Wei D, Zhao H, et al. 2014. Genotoxicity of quinolones:Substituents contribution and transformation products QSAR evaluation using 2D and 3D models[J]. Chemosphere, 95: 220–226.
DOI:10.1016/j.chemosphere.2013.09.002
|
Li W L, Zhang Z F, Ma W L, et al. 2018. An evaluation on the intra-day dynamics, seasonal variations and removal of selected pharmaceuticals and personal care products from urban wastewater treatment plants[J]. Science of the Total Environment, 640-641: 1139–1147.
DOI:10.1016/j.scitotenv.2018.05.362
|
Liu J, Lu G, Wang Y, et al. 2014. Bioconcentration, metabolism, and biomarker responses in freshwater fish Carassius auratus exposed to roxithromycin[J]. Chemosphere, 99: 102–108.
DOI:10.1016/j.chemosphere.2013.10.036
|
Lohwacharin J, Phetrak A, Takizawa S, et al. 2015. Bacterial growth during the start-up period of pilot-scale biological activated carbon filters:Effects of residual ozone and chlorine and backwash intervals[J]. Process Biochemistry, 50(10): 1640–1647.
DOI:10.1016/j.procbio.2015.06.012
|
Lu J, Fan L, Roddick F A. 2013. Potential of BAC combined with UVC/H2O2 for reducing organic matter from highly saline reverse osmosis concentrate produced from municipal wastewater reclamation[J]. Chemosphere, 93(4): 683–688.
DOI:10.1016/j.chemosphere.2013.06.008
|
刘成, 杨瑾涛, 李聪聪, 等. 2019. 生物活性炭在应用过程中的变化规律及其失效判定探讨[J]. 给水排水, 2019, 55(2): 9–16+21.
|
Lu Z, Sun W, Li C, et al. 2019. Bioremoval of non-steroidal anti-inflammatory drugs by Pseudoxanthomonas sp. DIN-3 isolated from biological activated carbon process[J]. Water Research, 161: 459–472.
DOI:10.1016/j.watres.2019.05.065
|
Luo Y, Guo W, Ngo H H, et al. 2014. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment[J]. Science of the Total Environment, 473-474: 619–641.
DOI:10.1016/j.scitotenv.2013.12.065
|
Ma X Y, Wang Y, Dong K, et al. 2019. The treatability of trace organic pollutants in WWTP effluent and associated biotoxicity reduction by advanced treatment processes for effluent quality improvement[J]. Water Research, 159: 423–433.
DOI:10.1016/j.watres.2019.05.011
|
Maier D, Blaha L, Giesy J P, et al. 2015. Biological plausibility as a tool to associate analytical data for micropollutants and effect potentials in wastewater, surface water, and sediments with effects in fishes[J]. Water Research, 72: 127–144.
DOI:10.1016/j.watres.2014.08.050
|
Mehrjouei M, Müller S, Möller D. 2015. A review on photocatalytic ozonation used for the treatment of water and wastewater[J]. Chemical Engineering Journal, 263: 209–219.
DOI:10.1016/j.cej.2014.10.112
|
Metz D H, Reynolds K, Meyer M, et al. 2011. The effect of UV/H2O2 treatment on biofilm formation potential[J]. Water Research, 45(2): 497–508.
DOI:10.1016/j.watres.2010.09.007
|
Miklos D B, Remy C, Jekel M, et al. 2018. Evaluation of advanced oxidation processes for water and wastewater treatment-A critical review[J]. Water Research, 139: 118–131.
DOI:10.1016/j.watres.2018.03.042
|
Mohapatra S, Huang C H, Mukherji S, et al. 2016. Occurrence and fate of pharmaceuticals in WWTPs in India and comparison with a similar study in the United States[J]. Chemosphere, 159: 526–535.
DOI:10.1016/j.chemosphere.2016.06.047
|
Palli L, Spina F, Varese G C, et al. 2019. Occurrence of selected pharmaceuticals in wastewater treatment plants of Tuscany:An effect-based approach to evaluate the potential environmental impact[J]. International Journal of Hygiene and Environmental Health, 222(4): 717–725.
DOI:10.1016/j.ijheh.2019.05.006
|
Papageorgiou M, Kosma C, Lambropoulou D. 2016. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece[J]. Science of the Total Environment, 543: 547–569.
DOI:10.1016/j.scitotenv.2015.11.047
|
Paredes L, Fernandez-Fontaina E, Lema J M, et al. 2016a. Understanding the fate of organic micropollutants in sand and granular activated carbon biofiltration systems[J]. Science of the Total Environment, 551-552: 640–648.
DOI:10.1016/j.scitotenv.2016.02.008
|
Paredes L, Fernandez-Fontaina E, Lema J M, et al. 2016b. Understanding the fate of organic micropollutants in sand and granular activated carbon biofiltration systems[J]. Science of the Total Environment, 551-552: 640–648.
DOI:10.1016/j.scitotenv.2016.02.008
|
Petrie B, Proctor K, Youdan J, et al. 2017. Critical evaluation of monitoring strategy for the multi-residue determination of 90 chiral and achiral micropollutants in effluent wastewater[J]. Science of the Total Environment, 579: 569–578.
DOI:10.1016/j.scitotenv.2016.11.059
|
Prasse C, Stalter D, Schulte-Oehlmann U, et al. 2015. Spoilt for choice:A critical review on the chemical and biological assessment of current wastewater treatment technologies[J]. Water Research, 87: 237–270.
DOI:10.1016/j.watres.2015.09.023
|
Pratt L A, Brody D J, Gu Q. 2017. Antidepressant use among persons aged 12 and over:United States, 2011-2014. NCHS Data Brief. Number 283[J]. National Center for Health Statistics, 283: 1–8.
|
Ramirez A J, Brain R A, Sascha U, et al. 2010. Occurrence of pharmaceuticals and personal care products in fish:results of a national pilot study in the United States[J]. Environmental Toxicology and Chemistry, 28(12): 2587–2597.
|
Reungoat J, Escher B I, Macova M, et al. 2012. Ozonation and biological activated carbon filtration of wastewater treatment plant effluents[J]. Water Research, 46(3): 863–872.
DOI:10.1016/j.watres.2011.11.064
|
Rivera-Jaimes J A, Postigo C, Melgoza-Alemán R M, et al. 2018. Study of pharmaceuticals in surface and wastewater from Cuernavaca, Morelos, Mexico:Occurrence and environmental risk assessment[J]. Science of the Total Environment, 613-614: 1263–1274.
DOI:10.1016/j.scitotenv.2017.09.134
|
Rodriguez-Mozaz S, Ricart M, Köck-Schulmeyer M, et al. 2015. Pharmaceuticals and pesticides in reclaimed water:Efficiency assessment of a microfiltration-reverse osmosis (MF-RO) pilot plant[J]. Journal of Hazardous Materials, 282: 165–173.
DOI:10.1016/j.jhazmat.2014.09.015
|
Rostvall A, Zhang W, Dürig W, et al. 2018. Removal of pharmaceuticals, perfluoroalkyl substances and other micropollutants from wastewater using lignite, Xylit, sand, granular activated carbon (GAC) and GAC+Polonite® in column tests-Role of physicochemical properties[J]. Water Research, 137: 97–106.
DOI:10.1016/j.watres.2018.03.008
|
Rozas O, Baeza C, Núñez K, et al. 2017. Organic micropollutants (OMPs) oxidation by ozone:Effect of activated carbon on toxicity abatement[J]. Science of the Total Environment, 590-591: 430–439.
DOI:10.1016/j.scitotenv.2016.12.120
|
Santos L H M L M, Gros M, Rodriguez-Mozaz S, et al. 2013. Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters:Identification of ecologically relevant pharmaceuticals[J]. Science of the Total Environment, 461-462: 302–316.
DOI:10.1016/j.scitotenv.2013.04.077
|
Sbardella L, Comas J, Fenu A, et al. 2018. Advanced biological activated carbon filter for removing pharmaceutically active compounds from treated wastewater[J]. Science of the Total Environment, 636: 519–529.
DOI:10.1016/j.scitotenv.2018.04.214
|
Scholz M, Martin R J. 1997. Ecological equilibrium on biological activated carbon[J]. Water Research, 31(12): 2959–2968.
DOI:10.1016/S0043-1354(97)00155-3
|
Semerjian L, Shanableh A, Semreen M H, et al. 2018. Human health risk assessment of pharmaceuticals in treated wastewater reused for non-potable applications in Sharjah, United Arab Emirates[J]. Environment International, 121: 325–331.
DOI:10.1016/j.envint.2018.08.048
|
Sharma B M, Bečanová J, Scheringer M, et al. 2019. Health and ecological risk assessment of emerging contaminants (pharmaceuticals, personal care products, and artificial sweeteners) in surface and groundwater (drinking water) in the Ganges River Basin, India[J]. Science of the Total Environment, 646: 1459–1467.
DOI:10.1016/j.scitotenv.2018.07.235
|
Sim W J, Lee J W, Oh J E. 2010. Occurrence and fate of pharmaceuticals in wastewater treatment plants and rivers in Korea[J]. Environmental Pollution, 158(5): 1938–1947.
DOI:10.1016/j.envpol.2009.10.036
|
Soltermann F, Abegglen C, Tschui M, et al. 2017. Options and limitations for bromate control during ozonation of wastewater[J]. Water Research, 116: 76–85.
DOI:10.1016/j.watres.2017.02.026
|
Souza M S, Hallgren P, Balseiro E, et al. 2013. Low concentrations, potential ecological consequences:Synthetic estrogens alter life-history and demographic structures of aquatic invertebrates[J]. Environmental Pollution, 178: 237–243.
DOI:10.1016/j.envpol.2013.03.038
|
Sun Y, Angelotti B, Brooks M, et al. 2018. A pilot-scale investigation of disinfection by-product precursors and trace organic removal mechanisms in ozone-biologically activated carbon treatment for potable reuse[J]. Chemosphere, 210: 539–549.
DOI:10.1016/j.chemosphere.2018.06.162
|
Suzuki T, Kosugi Y, Hosaka M, et al. 2014. Occurrence and behavior of the chiral anti-inflammatory drug naproxen in an aquatic environment[J]. Environmental Toxicology and Chemistry, 33(12): 2671–2678.
DOI:10.1002/etc.2741
|
Tarpani R R Z, Azapagic A. 2018. A methodology for estimating concentrations of pharmaceuticals and personal care products (PPCPs) in wastewater treatment plants and in freshwaters[J]. Science of the Total Environment, 622-623: 1417–1430.
DOI:10.1016/j.scitotenv.2017.12.059
|
Tewari S, Jindal R, Kho Y L, et al. 2013. Major pharmaceutical residues in wastewater treatment plants and receiving waters in Bangkok, Thailand, and associated ecological risks[J]. Chemosphere, 91(5): 697–704.
DOI:10.1016/j.chemosphere.2012.12.042
|
Thomaidi V S, Stasinakis A S, Borova V L, et al. 2015. Is there a risk for the aquatic environment due to the existence of emerging organic contaminants in treated domestic wastewater? Greece as a case-study[J]. Journal of Hazardous Materials, 283: 740–747.
DOI:10.1016/j.jhazmat.2014.10.023
|
Tran N H, Chen H, Reinhard M, et al. 2016. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes[J]. Water Research, 104: 461–472.
DOI:10.1016/j.watres.2016.08.040
|
Tran N H, Gin K Y H. 2017. Occurrence and removal of pharmaceuticals, hormones, personal care products, and endocrine disrupters in a full-scale water reclamation plant[J]. Science of the Total Environment, 599-600: 1503–1516.
DOI:10.1016/j.scitotenv.2017.05.097
|
Tran N H, Reinhard M, Gin K Y H. 2018. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review[J]. Water Research, 133: 182–207.
DOI:10.1016/j.watres.2017.12.029
|
Tran N H, Urase T, Ngo H H, et al. 2013. Insight into metabolic and cometabolic activities of autotrophic and heterotrophic microorganisms in the biodegradation of emerging trace organic contaminants[J]. Bioresource Technology, 146: 721–731.
DOI:10.1016/j.biortech.2013.07.083
|
Valizadeh B, Zokaee Ashtiani F, Fouladitajar A, et al. 2015. Scale-up economic assessment and experimental analysis of MF-RO integrated membrane systems in oily wastewater treatment plants for reuse application[J]. Desalination, 374: 31–37.
DOI:10.1016/j.desal.2015.07.017
|
Vinitnantharat S, Baral A, Ishibashi Y, et al. 2001. Quantitative bioregeneration of granular activated carbon loaded with phenol and 2, 4-dichlorophenol[J]. Environmental Technology, 22(3): 339–344.
DOI:10.1080/09593332208618288
|
Vulliet E, Cren-Olive C, Grenier-Loustalot M F. 2011. Occurrence of pharmaceuticals and hormones in drinking water treated from surface waters[J]. Environmental Chemistry Letters, 9(1): 103–114.
DOI:10.1007/s10311-009-0253-7
|
Wang F, van Halem D, Liu G, et al. 2017a. Effect of residual H2O2 from advanced oxidation processes on subsequent biological water treatment:A laboratory batch study[J]. Chemosphere, 185: 637–646.
DOI:10.1016/j.chemosphere.2017.07.073
|
Wang J, Tian Z, Huo Y, et al. 2018a. Monitoring of 943 organic micropollutants in wastewater from municipal wastewater treatment plants with secondary and advanced treatment processes[J]. Journal of Environmental Sciences, 67: 309–317.
DOI:10.1016/j.jes.2017.09.014
|
Wang L, Ying G G, Zhao J L, et al. 2010. Occurrence and risk assessment of acidic pharmaceuticals in the Yellow River, Hai River and Liao River of north China[J]. Science of the Total Environment, 408(16): 3139–3147.
DOI:10.1016/j.scitotenv.2010.04.047
|
Wang T, Huang Z X, Miao H F, et al. 2018b. Insights into influencing factor, degradation mechanism and potential toxicity involved in aqueous ozonation of oxcarbazepine (CHEM46939R1)[J]. Chemosphere, 201: 189–196.
DOI:10.1016/j.chemosphere.2018.02.062
|
Wang W L, Cai Y Z, Hu H Y, et al. 2019. Advanced treatment of bio-treated dyeing and finishing wastewater using ozone-biological activated carbon:A study on the synergistic effects[J]. Chemical Engineering Journal, 359: 168–175.
DOI:10.1016/j.cej.2018.11.059
|
Wang W L, Wu Q Y, Huang N, et al. 2018c. Potential risks from UV/H2O2 oxidation and UV photocatalysis:A review of toxic, assimilable, and sensory-unpleasant transformation products[J]. Water Research, 141: 109–125.
DOI:10.1016/j.watres.2018.05.005
|
Wang W L, Wu Q Y, Du Y, et al. 2017b. Elimination of chlorine-refractory carbamazepine by breakpoint chlorination:Reactive species and oxidation byproducts[J]. Water Research, 129: 115–122.
|
Wardenier N, Liu Z, Nikiforov A, et al. 2019. Micropollutant elimination by O3, UV and plasma-based AOPs:An evaluation of treatment and energy costs[J]. Chemosphere, 234: 715–724.
DOI:10.1016/j.chemosphere.2019.06.033
|
Wols B A, Hofman-Caris C H M. 2012. Modelling micropollutant degradation in UV/H2O2 systems:Lagrangian versus Eulerian method[J]. Chemical Engineering Journal, 210: 289–297.
DOI:10.1016/j.cej.2012.08.088
|
Xing L, Xie Y, Cao H, et al. 2014. Activated carbon-enhanced ozonation of oxalate attributed to HO oxidation in bulk solution and surface oxidation:Effects of the type and number of basic sites[J]. Chemical Engineering Journal, 245: 71–79.
DOI:10.1016/j.cej.2014.01.104
|
Xu J, Sun H, Zhang Y, et al. 2019. Occurrence and enantiomer profiles of β-blockers in wastewater and a receiving water body and adjacent soil in Tianjin, China[J]. Science of the Total Environment, 650: 1122–1130.
DOI:10.1016/j.scitotenv.2018.09.086
|
Xu N, Xu Y F, Xu S, et al. 2012. Removal of estrogens in municipal wastewater treatment plants:A Chinese perspective[J]. Environmental Pollution, 165: 215–224.
DOI:10.1016/j.envpol.2011.12.025
|
Yang J, Dong Z, Jiang C, et al. 2019. Quantitatively assessing the role played by carbonate radicals in bromate formation by ozonation[J]. Journal of Hazardous Materials, 363: 428–438.
DOI:10.1016/j.jhazmat.2018.10.013
|
Yang K, Yu J, Guo Q, et al. 2017a. Comparison of micropollutants' removal performance between pre-ozonation and post-ozonation using a pilot study[J]. Water Research, 111: 147–153.
DOI:10.1016/j.watres.2016.12.043
|
Yang X, Flowers R C, Weinberg H S, et al. 2011. Occurrence and removal of pharmaceuticals and personal care products (PPCPs) in an advanced wastewater reclamation plant[J]. Water Research, 45(16): 5218–5228.
DOI:10.1016/j.watres.2011.07.026
|
Yang Y Y, Liu W R, Liu Y S, et al. 2017b. Suitability of pharmaceuticals and personal care products (PPCPs) and artificial sweeteners (ASs) as wastewater indicators in the Pearl River Delta, South China[J]. Science of the Total Environment, 590-591: 611–619.
DOI:10.1016/j.scitotenv.2017.03.001
|
Yang Y, Ok Y S, Kim K H, et al. 2017c. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants:A review[J]. Science of The Total Environment, 596-597: 303–320.
DOI:10.1016/j.scitotenv.2017.04.102
|
Yi X, Tran N H, Yin T, et al. 2017. Removal of selected PPCPs, EDCs, and antibiotic resistance genes in landfill leachate by a full-scale constructed wetlands system[J]. Water Research, 121: 46–60.
DOI:10.1016/j.watres.2017.05.008
|
Yoon Y, Westerhoff P, Snyder S A, et al. 2006. Nanofiltration and ultrafiltration of endocrine disrupting compounds, pharmaceuticals and personal care products[J]. Journal of Membrane Science, 270(1): 88–100.
|
Yu Q, Geng J, Zong X, et al. 2019. Occurrence and removal of progestagens in municipal wastewater treatment plants from different regions in China[J]. Science of the Total Environment, 668: 1191–1199.
DOI:10.1016/j.scitotenv.2019.02.327
|
Zhang Y, Wang B, Cagnetta G, et al. 2018. Typical pharmaceuticals in major WWTPs in Beijing, China:Occurrence, load pattern and calculation reliability[J]. Water Research, 140: 291–300.
DOI:10.1016/j.watres.2018.04.056
|
Zhang Y, Zhuang Y, Geng J, et al. 2016. Reduction of antibiotic resistance genes in municipal wastewater effluent by advanced oxidation processes[J]. Science of The Total Environment, 550: 184–191.
DOI:10.1016/j.scitotenv.2016.01.078
|
Zoschke K, Börnick H, Worch E J W R. 2014. Vacuum-UV radiation at 185nm in water treatment:A review[J]. Water Research, 52(4): 131–145.
|
 2019, Vol. 39
2019, Vol. 39


