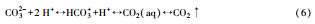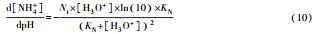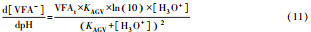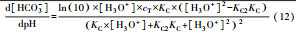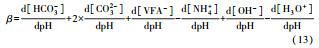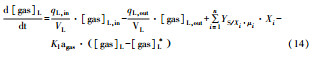2. 中国科学院生态环境研究中心, 水污染控制实验室, 北京 100085;
3. 中国科学院大学, 北京 100049
2. Department of Water Pollution Control Technology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085;
3. University of Chinese Academy of Sciences, Beijing 100049
京津冀地区2017年冬季的天然气荒引起广泛关注(中石油咨询中心, 2018), 表明我国亟需高效的清洁能源生产技术.厌氧消化是把有机污染物转化为清洁能源, 是极具前景的绿色技术, 例如将有机固体废弃物和高浓度有机废水转化为沼气(Vasco-Correa et al., 2018).“沼气升级(Biogas upgrading)”是指沼气中的甲烷浓度提升至天然气的80%以上, 便于进一步制备生物天然气(Vasco-Correa et al., 2018), 被认为是有机污染物极具潜力的资源化技术(Lee, 2017; Muñoz et al., 2015), 有助于我国能源结构优化和实现清洁低碳高效发展.据统计, 2016年丹麦生物天然气(1.8×108 m3)保障了10万户家庭供暖(Danish Energy Agency, 2017);研究表明, 2020年由沼气升级(Biogas upgrading)制备的生物天然气(Ullah Khan et al., 2017), 将供给欧洲可再生能源的25%以上(European Commission, 2016).目前沼气工程如污泥、畜禽养殖粪污(Chen et al., 2017)、农副食品加工废水(Yu et al., 2016)等厌氧处理的有机负荷(Organic loading rate, OLR)为2.1~8.6 kg · m-3 · d-1, 单位池容沼气产量为1.7~18.1 m3 · m-3 · d-1, 沼气中甲烷含量为47%~61%, 甲烷含量、容积负荷等关键技术性能有待提高(Chen and Liu, 2017; Ullah Khan et al., 2017);甲烷含量低则沼气热值低, 只能自用或发电, 难以制备生物天然气而实现高值化利用, 沼气经济效益差(Lee, 2017)限制了沼气工程的推广.因此, 提高沼气甲烷含量和厌氧消化负荷, 实现高负荷厌氧消化的沼气升级(制备生物天然气), 是我国应对清洁能源和有机污染物处理过程中迫切需要解决的重要技术问题.
高负荷厌氧消化以挥发性有机酸(VFAs)为主要中间产物生成甲烷, 伴随着VFAs等的底物消耗和氨氮(NH4+-N)等副产物的释放, 运行负荷主要受到酸化、抑制因子和微量元素等的影响和限制(Amha et al., 2018; Chen et al., 2016).高负荷厌氧消化及其抑制的一个共性特征是, VFAs、NH4+和碳酸盐等弱酸弱碱作为主要中间产物和副产物, 其变化贯穿于高负荷厌氧消化整个过程, 不仅使它们共同的pH可以作为高负荷厌氧消化有效的快速指征, 也使酸碱缓冲体系构建和调控成为高负荷厌氧消化的关键科学问题.然而, 高负荷厌氧消化的pH波动规律和成因, 及如何基于pH酸碱缓冲体系调控, 实现高负荷厌氧消化的沼气升级尚不清楚.本文在总结高负荷厌氧消化过程中pH变化规律和影响因素基础上, 分析了VFAs积累、氨氮抑制等不同机制下厌氧消化碳酸盐缓冲体系的特征, 及其对沼气中CH4/CO2构成的影响;并以厌氧膜生物反应器为例, 讨论了近年来厌氧消化基于pH在线监测和调控方法、相关理论模型方面的研究进展, 同时对未来的重点研究方向提出展望, 以期为今后高负荷厌氧生物反应器研发提供参考.
2 高负荷厌氧消化pH酸碱缓冲体系构成及其影响(Components and impacts of pH buffer system in high-rate anaerobic digestion)表 1中“水十条”十大行业中的农副食品加工业、原料药和畜禽养殖业产生的废水和废弃物往往具有高COD、高NH4+特征, 厌氧消化中VFAs酸化和NH4+抑制的风险并存(Khan et al., 2016; Ren et al., 2018).由于有机污染物浓度高, 导致中间产物如VFAs、NH4+等浓度均高于常规废水, 如食品加工废水厌氧处理过程中的NH4+可达700~3700 mg · L-1(表 1), 在弱酸弱碱构成中占较高比例.
| 表 1 高负荷厌氧消化的运行效果和酸碱缓冲体系* Table 1 Operating performance and acid-base buffer system of high load anaerobic digestion |
表 1显示, 出水pH往往高于进水pH(表 1), 高负荷厌氧消化VFAs大量消耗、氨氮不同程度释放是其重要原因.厌氧消化5个过程中(Wongnate et al., 2016), 底物水解酸化时释放大量的VFAs, 导致pH下降(Huang et al., 2016);pH下降幅度受到VFAs释放率、结构和碱度等的影响, 可能使pH < 6.0而酸化崩溃, 因而需要额外加碱调节(Yen et al., 2016).随着VFAs的消耗和副产物CO2的产生, pH回升;同时NH4+和HS-等逐步释放和积累, 可能产生氨氮抑制、pH过高等系列问题, 调控较复杂(Yuan and Zhu, 2016);甚至生物调控有时无法提供足够的酸度, 导致pH>8.5抑制产气, 也可能需要额外加酸降低pH值(Rico et al., 2017).
2.1 pH酸碱缓冲体系的主要构成现有研究认为厌氧消化产甲烷包括5个主要过程:水解(hydrolysis)、酸化(acidogenesis)、产氢产乙酸(syntrophic acetogenesis)、同型产乙酸(homoacetogenesis)和产甲烷(methanogenesis) (Xu et al., 2017).这5个过程中, VFAs、NH4+和碳酸盐等是其中重要的底物和副产物, 也是厌氧消化酸碱体系中主要变化的弱酸弱碱, 共同构成了厌氧消化过程中的酸碱缓冲体系(图 1).
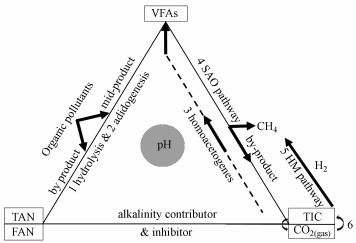 |
| 图 1 VFAs、NH4+和碳酸盐构成的酸碱缓冲体系及其形态变化 Fig. 1 Changes of pH buffer system formed by VFAs, NH4+ and carbonate |
在pH波动过程中, 这些底物和副产物在水中的化学形态也相应地发生变化和平衡移动(Salis and Monduzzi, 2016), 并影响产甲烷古菌的代谢途径、群落结构和产气性能(图 1).例如, 自由氨NH3向胞内扩散, 在胞内与NADH(nicotinamide adenine dinucleotide)形成质子竞争, 从而抑制了产甲烷过程(Amha et al., 2018).在产甲烷的途径4和途径5中, 强化途径5(并推动平衡6向CO2溶解移动), 理论上能够提高沼气中甲烷占比.但在厌氧消化的酸碱缓冲体系中, 以往多关注“NH4+”的抑制及“VFAs-碳酸盐碱度”酸碱体系(Wang et al., 2016; 2017), 而忽略了NH4+对碱度的贡献、CO2的气液平衡及其与生物过程的关系, 对“VFAs-NH4+-碳酸盐”三元缓冲体系形成过程缺乏深入研究.而该缓冲体系的变化过程对高效产甲烷, 从而提高沼气中CH4含量实现“沼气升级”至关重要.
2.2 酸碱缓冲体系构成对pH变化过程的影响为了描述厌氧消化的pH变化过程, IWA在ADM模型中包括了一个pH和碱度模块(Batstone et al., 2002; Hinken et al., 2014; Lovato et al., 2017), 其中采用双膜理论来描述气液传质, 并假设NH3/NH4+和CO2/HCO3-能够在厌氧消化过程中达到完全的气液平衡.根据厌氧消化产甲烷适宜的pH∈(6.5, 8.0)范围, 其碱度主要是碳酸氢盐构成, 例如蔗糖等农副食品加工废水的碱度通常 < 3000 mg · L-1(Yen et al., 2016), 而养殖废水由于固体中碱度的释放, 碱度可达12000 mg · L-1 (Rico et al., 2017).在生物过程之外, 有时矿物质和矿物质碱度也会对高负荷厌氧消化的pH产生较大的影响, 例如Ca2+或酒精废水中的S2-(Lu et al., 2017).其中, 挥发性VFAs、NH4+和碳酸盐又有电离平衡、形态转化、降解过程和相互作用, 三者的理化和生物过程较为复杂(Fang and Zhang, 2015; Zhang et al., 2015), 机理有待进一步明确, 因此, 相关的许多过程被ADM模型所忽略(Batstone et al., 2002).例如, NO3-还原为NH4+的竞争(Antwi et al., 2017b)、CaCO3等固体沉淀物对碱度的影响及弱酸和弱碱的抑制等(Li et al., 2018).这些被忽略的过程与酸碱缓冲体系密切相关, 而且很多逐渐成为目前厌氧消化动力学领域的研究热点.目前厌氧消化的重要关注点是电子转移、传递和竞争机理, 质子条件对工艺运行具有重要意义, 其酸碱缓冲体系机理尚不清楚.
在高负荷厌氧消化中, 通常VFAs、NH4+和碳酸盐浓度(318~3700 mg · L-1)远大于S2-浓度(26~385 mg · L-1), S2-对高负荷厌氧消化pH波动和碱度的贡献较小(Huang et al., 2015; Ward et al., 2011).同时, 由VFAs、NH4+和碳酸盐三元缓冲体系确定的pH值, 与淀粉废水、畜禽养殖粪污和混和厌氧消化等的高负荷厌氧消化试验结果相吻合(图 2).综上, 模型和初步试验都支持把高负荷厌氧消化的酸碱缓冲体系, 简化为由VFAs、NH4+和碳酸盐构成的三元pH酸碱缓冲体系, 从而深入考察高负荷厌氧消化碳酸盐缓冲体系的形成机制、特征及对微生物的影响.
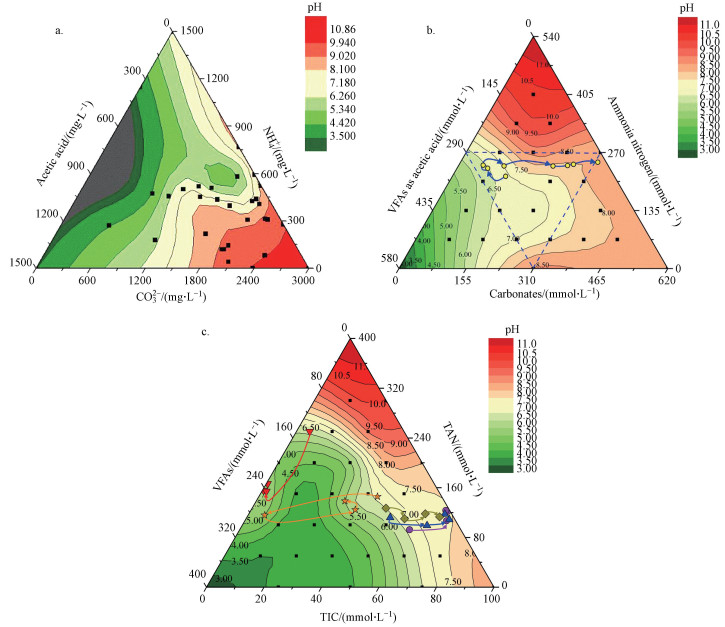 |
| 图 2 不同废水高负荷厌氧消化中的酸碱缓冲体系及其pH值三相图(a.淀粉废水(Yu et al., 2016), b.养殖粪污(Meng et al., 2018), c.混合厌氧消化(王子月等, 2018)) Fig. 2 pH of ternary acid-base buffer system in high load anaerobic digestion treating different wastewater (a. starch wastewater (Yu et al., 2016), b. manure slurry (Meng et al., 2018), c. co-digestion (Wang et al., 2018)) |
根据VFAs底物不同浓度的产甲烷速率特征, 按照米-门方程(Michaelis-Menten equation)分为3个典型阶段:①第一阶段的零级反应;②第二阶段的一级反应;③第三阶段的低速反应(Olsson, 2012).第一阶段由于VFAs转化为甲烷的消耗速率已经达到系统的上限, VFAs的累积将主要由VFAs释放速率决定(Wang and Chen, 2015);因此, 第一阶段VFAs和NH4+释放速率不同, 将决定第二阶段的主要限速因素是VFAs或NH4+, 从而形成了第二阶段的两个主要风险及其对应的两种三元缓冲体系:①VFAs积累(型);②氨氮抑制(型).
3.1 VFAs积累VFAs快速释放并积累而导致的产甲烷相酸化, 是高负荷厌氧消化处理富含易降解物的首要风险, 存在易酸化、出水COD和负荷的相互制约等问题(Li et al., 2018).农副食品加工业废水高负荷厌氧消化中VFAs 900~4000 mg · L-1, pH 4.2~7.2, 超过产甲烷pH 6.5~8.0的适宜范围, 导致负荷受限或系统酸化崩溃(Braguglia et al., 2018).同时, 农副食品加工业废水由于季节性生产和原料原因, 水质波动大导致系统负荷波动大(Meng et al., 2009), 对高负荷稳定运行的挑战较大.VFAs积累引起的pH波动程度主要取决于碱度, 常用VFAs和碱度ALK之间的比值(α=VFAs/ALK), 或不同碱度间关系(bicarbonate alkalinity to total alkalinity (BA/TA))来表征稳定性, 一般认为α < 0.3为稳定系统(Li et al., 2018).
VFAs中乙酸、丙酸、丁酸和戊酸等的比例结构对其降解速率有明显的影响.在升流式高负荷厌氧消化反应器中, 表现为沿升流方向有不同浓度梯度(Lima et al., 2016; Zhao et al., 2016).例如, 乙酸的降解速度高于丙酸20%~50%.因此, 丙酸更易累积, 丙酸与乙酸之比低于1.4时, 厌氧消化更易稳定运行(Luo et al., 2016).对于同样摩尔数的有机污染物, 由于丙酸的分子量比乙酸大23%, 电离常数更小, 从而贡献质子少, 导致pH降低的能力要低40%.对于长链脂肪酸(long-chain fatty acids, LCFAs)、S2-和重金属等导致的抑制, 这种自我调节机制避免了pH快速降低, 有利于高负荷厌氧消化稳定运行.但在NH4+等抑制引起的VFAs积累中, 反而不利于pH和NH3的迅速降低, 从而VFAs(特别是丙酸)-NH4+形成“受抑制的平衡态(inhibited steady-state)”(Fotidis et al., 2014; Tian et al., 2017).因此, NH4+抑制引起的VFAs相对积累量会更大, 导致系统负荷和COD去除率维持在较低的水平, 这是沼气产量损失的重要原因.
3.2 氨氮抑制及其对碱度的贡献明确氨氮抑制的机理有助于研发针对性的氨氮抑制缓解策略.一般认为, 自由氨(free ammonia, FAN)比NH4+更容易对微生物产生抑制作用, 且抑制程度更大, 是氨氮抑制的主要原因(Fotidis et al., 2017).NH3从底物中快速释放时, 更易导致高负荷厌氧消化系统的pH升高, 形成更多的FAN.FAN可直接进入胞内, 与胞内的NADH形成质子竞争;同时, 可能结合胞内H+, 导致胞内pH改变和K+不足, 降低了产甲烷菌活性(Sprott and Patel, 1986).NH3的半抑制浓度IC50远低于NH4+, 这同样说明NH3比NH4+更容易对产甲烷过程形成抑制作用(Poirier et al., 2016).
而Lay等的结果表明, 产甲烷菌的活性取决于NH4+的浓度, 而不是NH3的浓度, 驯化在其中发挥重要作用(Lay et al., 1998).同时, 高浓度NH4+会影响产甲烷菌合成甲烷的途径, 例如抑制Methanosaeta, 进而影响总体产甲烷效果(Amha et al., 2018).
内源性的NH4+释放对pH的影响普遍存在.根据化学平衡核算可知, 1 g氨氮(以N计)贡献3.57 g碳酸盐碱度(以CaCO3计)(Fang and Zhang, 2015).当NH4+浓度 < 2000 mg · L-1时, 对碱度的贡献较小, 可以忽略(Ho and Ho, 2012);当NH4+浓度>2700 mg · L-1时, 在中温厌氧消化pH>7.5或者高温厌氧消化中, 相应的NH3浓度从300 mg · L-1迅速升高, 且逐步释放的氨氮浓度升高导致pH升高、进一步导致NH3升高, 形成抑制的自我强化, 氨氮对碱度的贡献不可忽略(张玉秀等, 2018).例如, 养殖粪污中温厌氧消化, 当NH4+为4000 mg · L-1、pH=8.5时, NH3为1200 mg · L-1, 贡献碱度10%以上(Rico et al., 2017).
4 厌氧消化过程的碳酸盐缓冲体系(Carbonate buffer in anaerobic digestion)表 1说明, 出水pH越高, 沼气中的甲烷含量可能越高.高负荷厌氧消化与沼气中CO2的接触停留时间为3.5~48 h(Luo et al., 2016), 气液交换可接近或达到平衡, 更接近开放系统(Strumn et al., 1984), 而非传统认为的封闭系统.其不同于封闭系统的特征是, pH升高时碳酸盐碱度随之升高, 因而更能缓冲VFAs和氨氮浓度波动引起的pH变化;更重要的是当pH>~8.3时, 强化气液平衡, 沼气能够实现CO2≤~17%和CH4>80% (Wu et al., 2016), 并减少沼气中的H2S(表 1);同时, CO2为底物的氢营养型(hydrogenotrophic methanogenesis, HM)能适应更高的pH值(Poirier et al., 2016).因此, 不同于有固体存在的天然水体碳酸盐缓冲体系开放系统, 厌氧消化的碳酸盐缓冲体系是受生物调控的半开放系统, 明确其调控机制有助于维持pH和产气稳定, 拓宽高负荷厌氧消化的pH范围, 从而提高沼气甲烷含量, 实现“沼气升级”.
4.1 厌氧消化过程中碳酸盐缓冲体系的平衡特征由于空气中存在约0.29%的CO2, 其在水中的溶解和电离平衡导致地表水体中形成了广泛存在的碳酸盐缓冲体系, 其化学形态分布如图 3所示(Strumn et al., 1984).厌氧消化中碳酸盐缓冲体系的化学形态分布与地表水体类似, 但CO2aq浓度受气液平衡速度的影响较大(Lemmer et al., 2017).厌氧消化通常pH∈(4.5, 8.3), 根据图 3的碳酸盐缓冲体系形态与pH关系图, 其主要形态为HCO3-、H2CO3(G,亦即TIC).
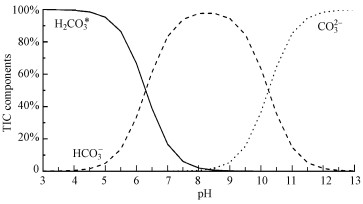 |
| 图 3 碳酸盐缓冲体系化学形态分布图 Fig. 3 Chemical components of carbonate buffer system |
当反应器停留时间较短(0.1~5 h)时, 可不考虑CO2气液平衡, 近似为封闭系统;停留时间较长时, 则为CO2气液平衡的开放系统, 如地表水平衡时间为2~19 d(Strumn et al., 1984).Li等研究了在产甲烷所需的最低碱度, 明确了气液平衡条件下碱度、pH与CO2分压关系(式(1)~(2))(Fang and Zhang, 2015).高负荷厌氧消化的停留时间一般为3.5~48 h(表 1), 为部分平衡的半开放碳酸盐缓冲系统.由于沼气中的CO2分压达21~99 kPa, 是空气中的CO2分压0.3 kPa的70~330倍, 气液平衡对沼气中甲烷含量影响大.
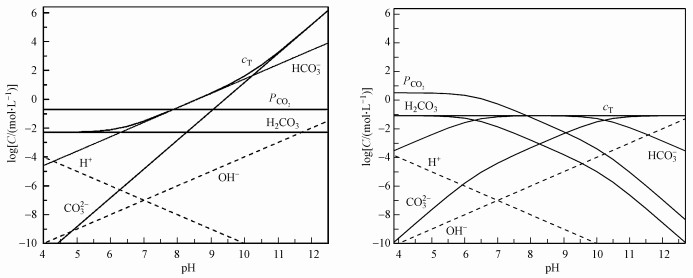 |
| 图 4 碳酸盐缓冲体系pH~log(c)形态图(a.开放系统, 37 ℃, Pco2=0.2 atm; b.封闭系统, 37 ℃, cT=1000 mg · L-1) Fig. 4 pH~log(c) relationship in carbonate buffer system(a. Open system, 37 ℃, Pco2=0.2 atm; b.close system, 37 ℃, cT=1000 mg · L-1) |
根据双膜扩散模型(Lemmer et al., 2017), CO2气液传质速率受气液界面对流强度、传质系数和浓度梯度影响.因此, 大量操作条件可以用来调控沼气中的CO2含量, 例如表面曝气、温度、pH和碱度, 乃至沼气压力(运行压力)等.
4.2 产甲烷过程对碳酸盐缓冲体系的影响CO2是产甲烷过程中的重要副产物和底物, 广泛参与厌氧消化产甲烷过程.其中, 丙酸产乙酸(acetogenesis, 式(1))、乙醇产乙酸(式(2))、乙酸产甲烷(methanogenesis, 式(3))、CO2产乙酸(homoacetogenesis, 式(4))、CO2产甲烷(式(5))等过程都涉及到CO2和碳酸盐(Wang et al., 2013), 与碳酸盐缓冲体系相互影响较大.
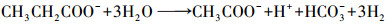
|
(1) |

|
(2) |

|
(3) |
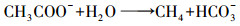
|
(4) |

|
(5) |
现有研究已表明提高H2分压有助于强化Acetyl-CoA pathway(Hao et al., 2012), 强化同型产乙酸(3, 图 1)和氢营养型产甲烷(5, 图 1), 有助于提高沼气中甲烷含量(Kougias et al., 2017; Lovato et al., 2017).根据相同的平衡移动原理, 通过提高总压而提高CO2分压等措施(Lemmer et al., 2017), 有可能实现类似效果.
| 表 2 厌氧消化过程中酸碱缓冲体系的变化及其相关动力学参数 Table 2 The change of pH buffer system and its kinetic parameters in anaerobic digestion |
在线自动控制是调控高负荷厌氧消化的酸碱缓冲体系、拓宽高效产甲烷pH范围的有效途径, 其通常由在线监测参数和自动控制策略构成(Nguyen et al., 2015).优选的在线监测参数应该能够准确、稳定和便于维护, 有效反映AnMBR的运行状态, 但由于高负荷厌氧消化的水质波动大、预处理复杂, 给其在线监测带来较大挑战(Li et al., 2018), 发展出许多巧妙的间接或近似监测方法, 以及不同工况和运行目标的自控策略.
5.1 基于pH的缓冲体系在线监测与预警现有研究开发了在线色谱(Motteran et al., 2017)、pH滴定(Mu et al., 2018)、谱学(Stockl and Lichti, 2018)和燃料电池(Yu et al., 2018)等厌氧消化的多参数在线监测方法.工程实践中应用的主要难点在于厌氧消化样品复杂的在线预处理, 而滴定方法无需样品预处理, 成为大型沼气工厂较为常用的在线监测方法(Lützhøft et al., 2014), 通过2点、3点或5点等pH多点滴定法, 基于缓冲体系推导的简单公式, 能够快速地计算出特定系统的VFAs、碳酸盐和氨氮等的浓度.然而, VFAs的构成、碳酸盐缓冲体系的气液交换等, 对滴定准确性的影响较大, 仍然是目前在线滴定法的主要难点(Mu et al., 2018).同时, pH和ORP还能反映产甲烷途径和其他优势(Salis and Monduzzi, 2016).
与在线系统较为复杂的液相监控相比, 沼气的流量及组分在线监控方案更为成熟(Bierer et al., 2016; Selvaraj et al., 2017), 因此, 气液两相联合监控使采用简单指标反映反应器状况成为可能(Yu et al., 2016).例如, 基于气相甲烷产气速率和液相VFA浓度差分曲线, 实现了原位“biogas upgrading”(Lovato et al., 2017).现有研究通过pH和Biogas的缓冲体系关系, 已经实现了NH4+、VFAs和碳酸盐的有效在线监测(Charnier et al., 2016), 并把高负荷厌氧消化稳定运行的pH范围拓展到了pH∈(6.2, 8.5)(Khan et al., 2016; Yu et al., 2016).
5.2 基于pH监测的在线控制策略厌氧消化控制策略传统上大多基于经验的黑箱控制模型, 例如阈值法、差分指数法(Dong et al., 2011)和模糊逻辑法(Antwi et al., 2018)等, 而基于知识模型或动力学模型的方法潜力较大.厌氧消化过程监控技术(process analytical technologies, PAT)的发展, 有赖于在线监测技术的进步和人们对厌氧消化动力学过程的深刻理解(Nguyen et al., 2015).
从反应速率的角度来考虑, 第一阶段VFAs和NH4+释放速率的相对关系, 驱动厌氧消化的pH波动, 从而改变CO2气液平衡和沼气甲烷浓度(表 1, 出水pH较高时甲烷浓度也较高), 最终影响产甲烷动力学速率, 并推动产甲烷代谢途径变化.根据该动力学过程形成的产气速率三阶段特征, 现有研究提出了“Biogas-pH”控制策略, 从而有效提高了系统负荷和甲烷产率, 并探讨了氨氮对其pH酸碱缓冲体系的作用(郁达伟, 2016).与VFAs驱动pH值和碱度变化的过程类似, 碱度在线滴定过程变化也是由酸驱动.现有研究建立了滴定过程中H3O+、OH-、NH4+、VFAs、HCO3-与pH动态关系的微分控制方程(Zaher, 2005), 即式(8)~(12);区别于基于平衡状态的稳态模型, 该微分方程组为瞬态模型方程组, 需要补充1个方程实现封闭求解后, 可用于在线监测.例如, 采用滴定过程定量外加酸, 可以用式(13)描述, 从而据此开发了pH和碱度的厌氧消化在线滴定系统(Charnier et al., 2016).如果采用产气动力学方程替代滴定方程, 把内源VFAs以产气动力学的QCH4=f(μ, pH, [H2], [NH4+], [LCFAs], [VFAs]), 通过沼气中CH4在线监测也可以封闭方程组, 实现基于Biogas-pH策略的气液两相监控和6组分在线监测(Yu et al., 2016).结合组分在线监测和碳酸盐缓冲体系定向调控策略, 通过CO2分压调控和生物驯化强化HM产甲烷途径, 使厌氧消化运行在较高pH条件下, 有望原位实现高热值的“沼气升级”.
6 结论(Conclusions)VFAs、NH4+和碳酸盐是高负荷厌氧消化重要的中间产物和副产物, 构成了高负荷厌氧消化酸碱缓冲体系主要的可变、可控部分.VFAs与NH4+的相对关系, 决定了高负荷厌氧消化产甲烷的pH趋势和抑制因子, 驱动着碳酸盐缓冲体系和系统中CO2的逸出, 形成半开放、受生物和底物影响的厌氧消化碳酸盐缓冲体系, 从而影响产甲烷途径和功能菌群的长期演替.三元缓冲体系贯穿高负荷厌氧消化整个过程, 对高负荷厌氧消化的稳定性、运行策略、负荷、甲烷产率和其浓度均有显著影响, 然而这些影响在高负荷厌氧消化中的特征尚有待研究.三元缓冲体系的形成机制和特征仍然有待明确, 适用于AnMBR等高负荷厌氧消化的缓冲体系调控措施也亟待研发, 从而为AnMBR等高负荷厌氧消化的“沼气升级”提供科技支撑, 为我国清洁能源生产和有机污染物处理提供技术保障.
Amha Y M, Anwar M Z, Brower A, et al. 2018. Inhibition of anaerobic digestion processes:Applications of molecular tools[J]. Bioresource Technology, 247: 999–1014.
DOI:10.1016/j.biortech.2017.08.210
|
Antwi P, Li J, Boadi P O, et al. 2017. Efficiency of an upflow anaerobic sludge blanket reactor treating potato starch processing wastewater and related process kinetics, functional microbial community and sludge morphology[J]. Bioresource Technology, 239: 105–116.
DOI:10.1016/j.biortech.2017.04.124
|
Antwi P, Li J, Boadi P O, et al. 2017b. Estimation of biogas and methane yields in an UASB treating potato starch processing wastewater with backpropagation artificial neural network[J]. Bioresource Technology, 228: 106–115.
DOI:10.1016/j.biortech.2016.12.045
|
Antwi P, Li J, Meng J, et al. 2018. Feedforward neural network model estimating pollutant removal process within mesophilic upflow anaerobic sludge blanket bioreactor treating industrial starch processing wastewater[J]. Bioresource Technology, 257: 102–112.
DOI:10.1016/j.biortech.2018.02.071
|
Batstone D J, Keller J, Angelidaki I, et al. 2002. The IWA Anaerobic Digestion Model No 1 (ADM1)[J]. Water Science and Technology, 45: 65–73.
|
Bierer B, Perez A O, Wöllenstein J, et al. 2016. In-situ biogas sensing system for enabling spatially resolved online determination of the gas composition of the fermenter[J]. Procedia Engeneer, 168: 1634–1637.
DOI:10.1016/j.proeng.2016.11.478
|
Braguglia C M, Gallipoli A, Gianico A, et al. 2018. Anaerobic bioconversion of food waste into energy:A critical review[J]. Bioresource Technology, 248: 37–56.
DOI:10.1016/j.biortech.2017.06.145
|
Charnier C, Latrille E, Lardon L, et al. 2016. Combining pH and electrical conductivity measurements to improve titrimetric methods to determine ammonia nitrogen, volatile fatty acids and inorganic carbon concentrations[J]. Water Research, 95: 268–279.
DOI:10.1016/j.watres.2016.03.017
|
Chen C, Guo W, Ngo H H, et al. 2016. Challenges in biogas production from anaerobic membrane bioreactors[J]. Renew Energy, 98: 120–134.
DOI:10.1016/j.renene.2016.03.095
|
Chen L, Cong R G, Shu B, et al. 2017. A sustainable biogas model in China:The case study of Beijing Deqingyuan biogas project[J]. Renewable & Sustainable Energy Reviews, 78: 773–779.
|
Chen Q, Liu T. 2017. Biogas system in rural China:Upgrading from decentralized to centralized? Renew[J]. Renewable & Sustainable Energy Reviews, 78: 933–944.
|
Danish Energy Agency, Ministry of Energy & Utilities and Climate, 2017. Biogas production in Denmark[R]. Copenhagen, 2-3
|
Dong F, Zhao Q B, Li W W, et al. 2011. Novel online monitoring and alert system for anaerobic digestion reactors[J]. Environmental Science and Technology, 45: 9093–9100.
DOI:10.1021/es202245f
|
European Commission, Directorate-general for energy, 2016. proposal for a directive of the european parliament and of the council on the promotion of the use of energy from renewable sources (recast)[R]. 2-5
|
Fang H H P, Zhang T. 2015. Anaerobic Biotechnology:Environmental Protection And Resource Recovery[M]. 1st ed. Imperial College Press, London.
|
Fotidis I A, Treu L, Angelidaki I. 2017. Enriched ammonia-tolerant methanogenic cultures as bioaugmentation inocula in continuous biomethanation processes[J]. Journal of Cleaner Production, 166: 1305–1313.
DOI:10.1016/j.jclepro.2017.08.151
|
Fotidis I A, Wang H, Fiedel N R, et al. 2014. Bioaugmentation as a solution to increase methane production from an ammonia-rich substrate[J]. Environmental Science and Technology, 48: 7669–7676.
DOI:10.1021/es5017075
|
Hao L P, Lü F, Li L, et al. 2012. Shift of pathways during initiation of thermophilic methanogenesis at different initial pH[J]. Bioresource Technology, 126: 418–424.
DOI:10.1016/j.biortech.2011.12.072
|
Hinken L, Huber M, Weichgrebe D, et al. 2014. Modified ADM1 for modelling an UASB reactor laboratory plant treating starch wastewater and synthetic substrate load tests[J]. Water Research, 64: 82–93.
DOI:10.1016/j.watres.2014.06.044
|
Ho L, Ho G. 2012. Mitigating ammonia inhibition of thermophilic anaerobic treatment of digested piggery wastewater:Use of pH reduction, zeolite, biomass and humic acid[J]. Water Research, 46: 4339–4350.
DOI:10.1016/j.watres.2012.05.016
|
Hu D, Su H, Chen Z, et al. 2017. Performance evaluation and microbial community dynamics in a novel AnMBR for treating antibiotic solvent wastewater[J]. Bioresource Technology, 243: 218–227.
DOI:10.1016/j.biortech.2017.06.095
|
Huang C, Zhao Y, Li Z, et al. 2015. Enhanced elementary sulfur recovery with sequential sulfate-reducing, denitrifying sulfide-oxidizing processes in a cylindrical-type anaerobic baffled reactor[J]. Bioresource Technology, 192: 478–485.
DOI:10.1016/j.biortech.2015.04.103
|
Huang W, Zhao Z, Yuan T, et al. 2016. Effective ammonia recovery from swine excreta through dry anaerobic digestion followed by ammonia stripping at high total solids content[J]. Biomass and Bioenergy, 90: 139–147.
DOI:10.1016/j.biombioe.2016.04.003
|
Khan M A, Ngo H H, Guo W S, et al. 2016. Comparing the value of bioproducts from different stages of anaerobic membrane bioreactors[J]. Bioresource Technology, 214: 816–825.
DOI:10.1016/j.biortech.2016.05.013
|
Kougias P G, Treu L, Benavente D P, et al. 2017. Ex-situ biogas upgrading and enhancement in different reactor systems[J]. Bioresource Technology, 225: 429–437.
DOI:10.1016/j.biortech.2016.11.124
|
Lay J J, Li Y Y, Noike T. 1998. The influence of pH and ammonia concentration on the methane production in high-solids digestion processes[J]. Water Environmental Research, 70: 1075–1082.
DOI:10.2175/106143098X123426
|
Lee D H. 2017. Evaluation the financial feasibility of biogas upgrading to biomethane, heat, CHP and AwR[J]. International Journal of Hydrogen Energy, 42: 27718–27731.
DOI:10.1016/j.ijhydene.2017.07.030
|
Lemmer A, Merkle W, Baer K, et al. 2017. Effects of high-pressure anaerobic digestion up to 30 bar on pH-value, production kinetics and specific methane yield[J]. Energy, 138: 659–667.
DOI:10.1016/j.energy.2017.07.095
|
Li L, Peng X, Wang X, et al. 2018. Anaerobic digestion of food waste:A review focusing on process stability[J]. Bioresource Technology, 248: 20–28.
DOI:10.1016/j.biortech.2017.07.012
|
Lima D M F, Rodrigues, J A D, et al. 2016. Anaerobic modeling for improving synergy and robustness of a manure co-digestion process[J]. Brazilian Journal Chemistry Engineering, 33: 871–883.
DOI:10.1590/0104-6632.20160334s20150314
|
Lovato G, Alvarado-Morales M, Kovalovszki A, et al. 2017. In-situ biogas upgrading process:Modeling and simulations aspects[J]. Bioresource Technology, 245: 332–341.
DOI:10.1016/j.biortech.2017.08.181
|
Lu X, Zhen G, Ni J, et al. 2017. Sulfidogenesis process to strengthen re-granulation for biodegradation of methanolic wastewater and microorganisms evolution in an UASB reactor[J]. Water Research, 108: 137–150.
DOI:10.1016/j.watres.2016.10.073
|
Luo G, Li J, Li Y, et al. 2016. Performance, kinetics behaviors and microbial community of internal circulation anaerobic reactor treating wastewater with high organic loading rate:Role of external hydraulic circulation[J]. Bioresource Technology, 222: 470–477.
DOI:10.1016/j.biortech.2016.10.023
|
Lützhøft H C H, Boe K, Fang C, et al. 2014. Comparison of VFA titration procedures used for monitoring the biogas process[J]. Water Research, 54: 262–272.
DOI:10.1016/j.watres.2014.02.001
|
Meng F, Chae S R, Drews A, et al. 2009. Recent advances in membrane bioreactors (MBRs):Membrane fouling and membrane material[J]. Water Research, 43: 1489–1512.
DOI:10.1016/j.watres.2008.12.044
|
Meng X S, Yu D W, Wei Y S, et al. 2018. Endogenous ternary pH buffer system with ammonia-carbonates-VFAs in high solid anaerobic digestion of swine manure:An alternative for alleviating ammonia inhibition?[J]. Process Biochemistry, 69: 144–152.
DOI:10.1016/j.procbio.2018.03.015
|
Mortezaei Y, Amani T, Elyasi S, et al. 2018. High-rate anaerobic digestion of yogurt wastewater in a hybrid EGSB and fixed-bed reactor:Optimizing through response surface methodology[J]. Process Safety & Environmental Protection, 113: 255–263.
|
Motteran F, Lima Gomes P C F, Silva E L, et al. 2017. Simultaneous determination of anionic and nonionic surfactants in commercial laundry wastewater and anaerobic fluidized bed reactor effluent by online column-switching liquid chromatography/tandem mass spectrometry[J]. Science of the Total Environment, 580(C): 1120–1128.
|
Mu Z X, He C S, Jiang J K, et al. 2018. A modified two-point titration method for the determination of volatile fatty acids in anaerobic systems[J]. Chemosphere, 204: 251–256.
DOI:10.1016/j.chemosphere.2018.04.038
|
Muñoz R, Meier L, Diaz I, et al. 2015. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading[J]. Reviews in Environmental Science & Bio/Technology, 14(4): 727–759.
|
Ng K K, Shi X, Ng H Y. 2015. Evaluation of system performance and microbial communities of abioaugmented anaerobic membrane bioreactor treating pharmaceutical wastewater[J]. Water Research, 81: 311–324.
DOI:10.1016/j.watres.2015.05.033
|
Nguyen D, Gadhamshetty V, Nitayavardhana S, et al. 2015. Automatic process control in anaerobic digestion technology:A critical review[J]. Bioresource Technology, 193: 513–522.
DOI:10.1016/j.biortech.2015.06.080
|
Olsson G. 2012. ICA and me-A subjective review[J]. Water Research, 46: 1585–1624.
DOI:10.1016/j.watres.2011.12.054
|
Poirier S, Desmond-Le Quéméner E, Madigou C, et al. 2016. Anaerobic digestion of biowaste under extreme ammonia concentration:Identification of key microbial phylotypes[J]. Bioresource Technology, 207: 92–101.
DOI:10.1016/j.biortech.2016.01.124
|
Ren Y, Yu M, Wu C, et al. 2018. A comprehensive review on food waste anaerobic digestion:Research updates and tendencies[J]. Bioresource Technology, 247: 1069–1076.
DOI:10.1016/j.biortech.2017.09.109
|
Rico C, Montes J A, Rico J L. 2017. Evaluation of different types of anaerobic seed sludge for the high rate anaerobic digestion of pig slurry in UASB reactors[J]. Bioresource Technology, 238: 147–156.
DOI:10.1016/j.biortech.2017.04.014
|
Salis A, Monduzzi M. 2016. Not only pH. specific buffer effects in biological systems[J]. Current Opinion in Colloid & Interface Science, 23: 1–9.
|
Selvaraj R, Abdul A N, Vasa N J, et al. 2017. Monitoring of CO2 and CH4 composition in a biogas matrix from different biomass structures. Sensors Actuators[J]. Bioprocess & Biosystems Engineering, 249: 378–385.
|
Sprott G D, Patel G B. 1986. Ammonia toxicity in pure cultures of methanogenic bacteria[J]. Systematic & Applied Microbiology, 7(2): 358–363.
|
Stockl A, Lichti F. 2018. Near-infrared spectroscopy (NIRS) for a real time monitoring of the biogas process[J]. Bioresource Technology, 247: 1249–1252.
DOI:10.1016/j.biortech.2017.09.173
|
Strumn W, Morgan J J, 汤鸿霄. 1984.水化学-天然水体化学平衡导论[M].北京: 科学出版社
|
Tian H, Fotidis I A, Mancini E, et al. 2017. Different cultivation methods to acclimatise ammonia-tolerant methanogenic consortia[J]. Bioresource Technology, 232: 1–9.
DOI:10.1016/j.biortech.2017.02.034
|
Ullah K I, Hafiz M H. 2017. Biogas as a renewable energy fuel-A review of biogas upgrading, utilisation and storage[J]. Energy Conversion & Management, 150: 277–294.
|
Vasco-Correa J, Khanal S, Manandhar A, et al. 2018. Anaerobic digestion for bioenergy production:Global status, environmental and techno-economic implications, and government policies[J]. Bioresource Technology, 247: 1015–1026.
DOI:10.1016/j.biortech.2017.09.004
|
Wang D, Chen Y. 2015. Critical review of the influences of nanoparticles on biological wastewater treatment and sludge digestion[J]. Critical Reviews in Biotechnology, 36(5): 816–828.
|
Wang H, Fotidis I A, Angelidaki I. 2016. Ammonia-LCFA synergetic co-inhibition effect in manure-based continuous biomethanation process[J]. Bioresource Technology, 209: 282–289.
DOI:10.1016/j.biortech.2016.03.003
|
Wang H, Tao Y, Gao D, et al. 2015. Microbial population dynamics in response to increasing loadings of pre-hydrolyzed pig manure in an expanded granular sludge bed[J]. Water Research, 87: 29–37.
DOI:10.1016/j.watres.2015.09.008
|
Wang J, Xu W, Yan J, et al. 2014. Study on the flow characteristics and the wastewater treatment performance in modified internal circulation reactor[J]. Chemosphere, 117: 631–637.
DOI:10.1016/j.chemosphere.2014.09.088
|
Wang W, Xie L, Luo G, et al. 2013. Performance and microbial community analysis of the anaerobic reactor with coke oven gas biomethanation and in situ biogas upgrading[J]. Bioresource Technology, 146: 234–239.
DOI:10.1016/j.biortech.2013.07.049
|
Wang Y, Zang B, Gong X, et al. 2017. Effects of pH buffering agents on the anaerobic hydrolysis acidification stage of kitchen waste[J]. Waste Management, 68: 603–609.
DOI:10.1016/j.wasman.2017.06.041
|
Ward A J, Bruni E, Lykkegaard M K, et al. 2011. Real time monitoring of a biogas digester with gas chromatography, near-infrared spectroscopy, and membrane-inlet mass spectrometry[J]. Bioresource Technology, 102: 4098–4103.
DOI:10.1016/j.biortech.2010.12.052
|
Wongnate T, Sliwa D, Ginovska B, et al. 2016. The radical mechanism of biological methane synthesis by methylcoenzyme M reductase[J]. Science, 352: 953–958.
DOI:10.1126/science.aaf0616
|
Wu Y, Wang C, Liu X, et al. 2016. A new method of two-phase anaerobic digestion for fruit and vegetable waste treatment[J]. Bioresource Technology, 211: 16–23.
DOI:10.1016/j.biortech.2016.03.050
|
王子月, 张长平, 孟晓山, 等. 2018. 猪粪与酒糟混合厌氧发酵的产甲烷和三元pH缓冲体系特征[J]. 环境工程学报, 2018, 8: 21–27.
|
Xu Q, Li X, Ding R, et al. 2017. Understanding and mitigating the toxicity of cadmium to the anaerobic fermentation of waste activated sludge[J]. Water Research, 124: 269–279.
DOI:10.1016/j.watres.2017.07.067
|
郁达伟. 2016.厌氧膜生物反应器处理高浓度有机废水及其优化研究[D].北京: 中国科学院生态环境研究中心
|
Yang B, Wang M, Wang J, et al. 2018. Mechanism of high contaminant removal performance in the expanded granular sludge blanket (EGSB) reactor involved with granular activated carbon for low-strength wastewater treatment[J]. Chemical Engineering Journal, 334: 1176–1185.
DOI:10.1016/j.cej.2017.11.072
|
Yen F C, Chang T C, Hu C C, et al. 2016. Feasibility of combined upflow anaerobic sludge blanket-aerobic membrane bioreactor system in treating purified terephthalic acid wastewater and polyimide membrane for biogas purification[J]. Journal of Environmental Chemical Engineering, 4(4): 4113–4119.
DOI:10.1016/j.jece.2016.09.010
|
Yu D, Liu J, Sui Q, et al. 2016. Biogas-pH automation control strategy for optimizing organic loading rate of anaerobic membrane bioreactor treating high COD wastewater[J]. Bioresource Technology, 203: 62–70.
DOI:10.1016/j.biortech.2015.12.010
|
Yu Z, Leng X, Zhao S, et al. 2018. A review on the applications of microbial electrolysis cells in anaerobic digestion[J]. Bioresource Technology, 255: 340–348.
DOI:10.1016/j.biortech.2018.02.003
|
Yuan H, Zhu N. 2016. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion[J]. Renewable & Sustainable Energy Reviews, 58: 429–438.
|
Zaher U. 2005. Modelling and monitoring the anaerobic digestion process in view of optimisation and smooth operation of WWTP's[D]. Ghent: Ghent University.
|
Zhang T, Mao C, Zhai N, et al. 2015. Influence of initial pH on thermophilic anaerobic co-digestion of swine manure and maize stalk[J]. Waste Management, 35: 119–126.
DOI:10.1016/j.wasman.2014.09.004
|
Zhao Y, Yu J, Liu J, et al. 2016. Material and microbial changes during corn stalk silage and their effects on methane fermentation[J]. Bioresource Technology, 222: 89–99.
DOI:10.1016/j.biortech.2016.09.113
|
中石油咨询中心. 2018. "气荒"的警示与对策建议[J]. 中国能源报, 2018, 125(3): 14.
|
张玉秀, 孟晓山, 王亚炜, 等. 2018. 畜禽废弃物厌氧消化过程的氨氮抑制及其应对措施研究进展[J]. 环境工程学报, 2018, 12(4): 985–998.
|
 2019, Vol. 39
2019, Vol. 39