苯酚及其衍生物是从许多行业(如制药、石油、石化、农药、塑料、造纸和其他制造业)排放出来的, 对人类和其它生物会产生严重危害(Park et al., 2013).酚及其衍生物因具有毒性并可在环境中积累, 从而成为受到高度优先关注的污染物(Huang et al., 2015).研究发现, 含酚废水的排放已对土壤/沉积物、地表水及地下水等造成了严重的污染(Lamar et al., 2010; 裴芳等, 2012; 尹勇等, 2012; Khairy, 2013; Chen et al., 2014; Zhou et al., 2017).持久性有机化合物在污染场地中的分布受土壤/沉积物的化学吸附行为的强烈影响(Kan, 1997), 酚类化合物进入水环境后, 更倾向于分配到土壤和沉积物中(Jackson et al., 1990), 例如, 某退役炼油厂污染场地土壤中挥发酚的富集量可高达2825.7 mg·kg-1(裴芳等, 2012).地质吸附剂(土壤/沉积物)对疏水性有机污染物的化学相互作用可能导致强结合和缓慢的后续释放速率, 并显著影响修复率和终点(Luthy et al., 1997).大量研究表明, 土壤或沉积物中的少量污染物可以很容易地解吸到水相中, 而其余部分的解吸则非常困难, 不遵循传统的解吸模型, 通常被称为“封存”(Tomson, 2006).被不可逆地吸附在土壤/沉积物中的这部分污染物, 无论是生物活性还是生物方法, 对它们都没有实际意义, 可以安全地留在原地(Kan et al., 1998).土壤/沉积物中的一部分污染物对降解微生物的污染程度低得多, 有研究将这种生物有效性的降低归因于土壤/沉积物对污染物的封存(Beckles et al., 2007).虽然这种降低的生物有效性往往降低了土壤和沉积物修复的有效性, 但同样也可以大大降低了污染土壤和沉积物的环境风险(Tomson, 2006).
有机碳和黏土矿物通常是控制有机化学品进入土壤环境的两个最重要的组分, 土壤中的非离子有机化合物容易被土壤有机质吸收(Zhang et al., 2011).同时, 有机质的性质和含量还决定了河口沉积物对疏水性有机污染物的吸附(Shang et al., 2013).研究表明, 土壤/沉积物颗粒中的有机质是吸附有机污染物的重要介质(Murphy et al., 1990; Murphy et al., 1994; Ghosh et al., 2000);土壤有机质有助于有机化学品的封存(Schaumann et al., 2010);残留在土壤中的菲绝大部分(>92%)与土壤腐殖质结合(Wang et al., 2017).在以前的研究中假定降解的多环芳烃有相当一部分可能已被封存在有机碳中(Northcott et al., 2001);菲和阿特拉津的封存与有机碳含量高度相关(Chung et al., 2002).研究发现, 自然条件下的腐殖质很少以单体形式存在, 常常与黏土矿物、氧化矿物相互结合形成有机-矿质复合体(Kaiser et al., 2000; Kalbitz et al., 2005; bKögel-knabner et al., 2008; Ksiezopolska et al., 2011).疏水性有机污染物在腐殖质中的吸附与腐殖质-矿质复合体中存在很大差异(Jones et al., 1999), 腐殖质被吸附到黏土矿物表面后, 其物理构象发生改变, 从而对疏水性有机污染物的吸附产生影响(Jones et al., 1999), 进而增强了对疏水性有机污染物的吸附能力(Terashima et al., 2003; Wang et al., 2005).土壤/沉积物对有机污染物的吸附实际上是土壤/沉积物中的有机矿质复合体在起作用(Huang et al., 1996; Mader et al., 1997), 有机-矿质复合体在调节疏水性有机污染物在土壤/沉积物中的迁移和滞留方面起着非常重要的作用(Wang et al.2005; Kang et al., 2008).土壤颗粒网状结构的形成对有机污染物的滞留、释放及运移都固有地受到与矿物基质结合的天然有机质(NOM)的影响(Tombácz et al., 2004).而NOM的主要组成部分是腐殖质, 可占到NOM的50%~80%(Shaker et al., 2012).研究发现, 土壤腐殖质可划分为富里酸(FA)、胡敏酸(HA)和胡敏素(HM)3个组分(lehtonen et al., 2001; Litvin et al., 2012; Han et al., 2016).腐殖质可通过Ca2+、Al3+、Fe3+等多价阳离子桥与黏土矿物相互作用形成有机-矿质复合体(Oades, 1988; Duchaufour, 1998; Yang et al., 2001).吸附和解吸是控制有机污染物在自然环境中运移和归趋的关键过程(Tomson, 2006).不可逆吸附(封存)对土壤环境中有机污染物(六氯环己烷、阿特拉津)的暴露和归趋有显著影响, 可以促进有机污染物(六氯环己烷、阿特拉津)污染场地的控制、管理和修复(Duan et al., 2008; Yang et al.2009).基于此, 本文通过平衡吸附/解吸试验方法, 研究不同腐殖质组分团聚体对苯酚封存特征的影响, 以期为利用腐殖质修复受苯酚污染场地土壤提供科学依据.
2 材料与方法(Materials and methods) 2.1 试验材料 2.1.1 腐殖质的提取及纯化草炭样品预处理(去除非腐殖质有机质):在索氏提取器中用苯/甲醇(2:1, V/V)提取草炭, 用0.1 mol·L-1 HCl处理剩余固体, 并用去离子水洗涤(Gondar et al., 2005; Shirshova et al., 2006).
HA和FA的提取、分离与纯化:按国际腐殖酸物质协会(IHSS)推荐的方法提取HA和FA, 在通入N2条件下以10:1(V/m, mL/g)的萃取剂(0.1 mol·L-1NaOH)与草炭比萃取, 然后用6 mol·L-1 HCl酸化提取物至pH=1, 将提取的腐殖质分离成HA和FA组分.沉淀(HA)和上清液(FA)通过离心分离(Gondar et al., 2005; Shirshova et al., 2006).
HA纯化:将HA组分悬浮在0.1 mol·L-1 HCl/0.3 mol·L-1 HF的溶液中以除去矿物杂质, 然后透析直到无Cl-, 样品冷冻干燥后备用(Gondar et al., 2005; Shirshova et al., 2006).
FA纯化:用吸附树脂XAD-8纯化, 碱性洗脱液通过H+饱和阳离子交换树脂, 去除阳离子, 样品冷冻干燥后备用(Shirshova et al., 2006; Gondar et al., 2005).
HM提取方法:称取提取FA和HA后的残渣5 g, 用浓HCl(12 mol·L-1)浸泡, 先后2次加入二甲基亚砜(分别为250、100 mL)提取.滴加饱和NaOH调节pH至8~9, 沉淀胡敏素, HCl酸化, 然后透析直到无Cl-, 样品冷冻干燥后备用(Tsutsuki et al.2012).
2.1.2 黏土矿物纯化有机-矿质复合体制备所用的2种黏土矿物(1:1型黏土矿物高岭石、2:1型黏土矿物蒙脱石)均为化学纯商品黏土矿物(购于国药集团化学试剂沈阳有限公司).
有机质的去除(过氧化氢氧化法):用30%过氧化氢去除黏土矿物中的有机质(Pan et al., 2010; Wei et al., 2014; Huang et al., 2005).
黏粒的提取:利用沉降法分离出每种黏土矿物的 < 2 μm级分(Huang et al., 2005; Zhang et al., 2011; Zhang et al., 2012; Wei et al., 2014).
矿物黏粒的钠化:将黏粒重悬浮在0.01 mol·L-1的NaCl中以去除可交换阳离子而分别使其均一化, 用去离子水重复离心洗涤除去过量的盐, 直到无Cl-, 冷冻干燥后备用(Tombácz et al., 2004; Zhang et al., 2012).
黏土矿物的钙、铁、铝化:分别称取50 g去除有机质和可交换阳离子后的 < 2 μm的黏土矿物(高岭石和蒙脱石黏粒)各3份, 分别放入500 mL 0.5 mol·L-1的氯化钙、氯化铝和氯化铁溶液中, 25 ℃恒温振荡浸泡7 d后, 冷冻干燥, 备用(Wang et al., 2005; 倪进治等, 2008; Zhang et al., 2011; Zhu et al., 2016).使黏土矿物分别被钙、铁、铝所饱和, 制备的钙、铁、铝化黏土矿物表面的钙、铁、铝氧化物用2 mol·L-1HCl洗涤去除(Zhang et al., 2012), 再用去离子水漂洗3次去除游离Cl-, 再次冷冻干燥后备用.
2.1.3 有机-矿质复合体的制备分别称取5 g FA、HA、HM各3份, FA、HA用50 mL 0.1 mol·L-1 NaOH助溶, HM用50 mL二甲基亚砜助溶, 每种腐殖质组分(3份)助溶后分别加入到500 mL 0.5 mol·L-1 CaCl2、AlCl3和FeCl3溶液中, 再分别将对应的钙、铁、铝化黏土矿物50 g加入到此溶液中.振荡浸泡7 d后, 将有机-矿质复合体样品冷冻干燥(Wang et al., 2005; 倪进治等, 2008;Zhang et al., 2011; Zhu X, et al., 2016; ).有机-矿质复合体中游离的腐殖质通过再悬浮、离心, 用去离子水洗涤以除去任何未结合的FA、HA和HM, 直到总有机碳分析时未观察到显著解吸(Zhang et al., 2012), 再次冷冻干燥后备用.Ca2+、Al3+、Fe3+桥键制备的高岭石、蒙脱石不同腐殖质组分团聚体见表 1.
| 表 1 Ca2+、Al3+、Fe3+桥键制备的高岭石、蒙脱石不同腐殖质组分团聚体 Table 1 Aggregates with different humus fraction under Ca2+、Al3+、Fe3+ bridge bond action |
苯酚标准溶液采用优级纯苯酚试剂配制, 吸附试验初始苯酚标准溶液浓度序列为10、20、30、40、50、60、70、80、90、100 mg·L-1(徐红霞等, 2008; 贺婧等, 2012; Ma et al., 2016; 任爽等, 2017), 配制时每升溶液中加入10 mL 1 mol·L-1 NaN3 (保持NaN3浓度在0.01 mol·L-1, 以抑制微生物对苯酚的降解)(贺婧等, 2012)及10 mL 1 mol·L-1 NaCl (保持NaCl离子强度在0.01 mg·L-1)(任爽等, 2017).
吸附试验:称取制备样品2.5000g, 置于100mL三角瓶中, 分别加入不同浓度苯酚标准溶液25mL, 固液比为1:10, 在恒温(20℃)振荡器上振荡吸附24 h, 静止平衡2 h, 上清液通过0.45 μm微孔滤膜后, 测定苯酚浓度.由初始苯酚浓度与平衡溶液苯酚浓度差值计算得出样品对苯酚的吸附量, 具体见式(1)(徐红霞等, 2008; 贺婧等, 2012; Ma et al., 2016; 任爽等, 2017).为保证数据质量, 设置3次重复, 取3次测定结果的平均值(变异系数小于5%).
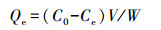
|
(1) |
式中, C0为苯酚初始浓度(mg·L-1);Ce为吸附平衡时苯酚浓度(mg·L-1);V为平衡溶液体积(mL);W为供试样品质量(g);Qe为吸附平衡时的吸附量(mg·kg-1).
2.2.2 解吸实验设计在样品对苯酚到达饱和吸附后的每个三角瓶中, 分别加入电解质溶液(NaCl离子强度0.01 mol·L-1(任爽等, 2017), NaN3浓度0.01 mol·L-1, 拟制微生物对苯酚的降解(贺婧等, 2012))25 mL, 固液比为1:10, 在恒温(20 ℃)振荡器上振荡解吸24 h, 静止平衡2 h, 上清液通过0.45 μm微孔滤膜后, 测定苯酚浓度, 由式(2)计算样品的苯酚解吸量(Ma et al., 2016).每个样品设置3次重复, 取用3次测定结果的平均值(变异系数小于5%).
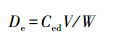
|
(2) |
式中, Ced为解吸平衡时的苯酚浓度(mg·L-1);V为平衡溶液体积(mL);W为供试样品质量(g);De为解吸平衡时的解吸量(mg·kg-1).
2.3 测试方法苯酚采用液相色谱(日本岛津LC-20AD)测定, 基本运行参数为:流动相为甲醇:水(75:25, 体积比), 流速为1.0 mL·min-1, 紫外检测器波长为284 nm, 进样量为20 μL, 外标法定量(徐红霞等, 2008);团聚体有机碳质量分数采用水合热重铬酸钾氧化-比色法测定(鲁如坤, 2000);团聚体总比表面积采用乙二醇乙醚吸附法(EGME)测定(鲁如坤, 2000);团聚体外比表面积采用三乙胺阳离子(TEA)饱和、乙二醇乙醚吸附法(EGME)测定(鲁如坤, 2000);团聚体内比表面积由总比表面积减外比表面积计算得出(鲁如坤, 2000);团聚体Fe2O3、Al2O3、CaO含量采用X衍射分析.
2.4 计算方法 2.4.1 吸附分配系数不同腐殖质组分团聚体对苯酚的吸附特征用Freundlich吸附方程来定量描述:
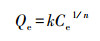
|
(3) |
式中, Qe为吸附平衡时的吸附量(mg·kg-1);Ce为吸附平衡时液相中的吸附质浓度(mg·L-1);k为吸附分配系数, 是指在一定平衡溶液浓度条件下, 吸附质在固相和液相中的分配比, 可直观表征吸附剂对吸附质的吸附容量的大小;n为吸附速率常数, 表示随着吸附质溶液浓度的增加, 吸附量增加的速度.式(3)直线化可得:lnQe=lnk+(1/n)lnCe, 以lnQe对lnCe作图, 即可求得各特征值.
2.4.2 饱和吸附量不同腐殖质组分团聚体对苯酚的饱和吸附量由Langmuir吸附方程(Carvalho et al., 2012; Huang et al., 2015; Ma et al., 2016)计算得出:
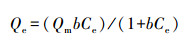
|
(4) |
式中, Qe为吸附平衡时的吸附量(mg·kg-1);Ce为吸附平衡时液相中的苯酚浓度(mg·L-1);b为吸附作用的平衡常数;Qm为饱和吸附量(mg·kg-1).
2.4.3 解吸分配系数不同腐殖质组分团聚体对苯酚的解吸特征用Freundlich解吸方程来定量描述:
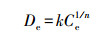
|
(5) |
式中, De为解吸平衡时的解吸量(mg·kg-1);Ced为解吸平衡时液相中的吸附质浓度(mg·L-1);kd为解吸分配系数, 表示在一定平衡溶液浓度条件下, 吸附质在固相和液相中的分配比, 可直观表征吸附剂对吸附质的解吸附能力的大小;n为解吸速率常数, 表示随着吸附质溶液浓度的降低, 解吸量降低的速度.
2.4.4 最大解吸量不同腐殖质组分团聚体对苯酚的最大解吸量由Langmuir解吸方程(Ma et al., 2016)计算得出:

|
(6) |
式中, De为解吸平衡时的解吸量(mg·kg-1);Ced为解吸平衡时液相中的苯酚浓度(mg·L-1);bd为解吸作用的平衡常数;Dm为苯酚接近完全解吸时的解吸量(mg·kg-1).
2.4.5 不可逆吸附量和封存系数土壤/沉积物中同时存在着可逆吸附室和不可逆吸附室, 污染物在两室中的吸附机制是不同的, 解吸只发生在可逆吸附室中(Kan et al., 1997; Kan et al., 1998).不同腐殖质组分团聚体对苯酚的不可逆吸附量Qmirr(mg·kg-1)用(7)式计算, 封存系数用式(8)计算.
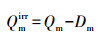
|
(7) |

|
(8) |
式中, SR为封存系数, SR值越大, 表示团聚体对苯酚的封存能力越强.
3 结果与分析(Results and analysis) 3.1 供试团聚体理化性质供试团聚体的理化性质见表 2, 可以看出, 高岭石不同腐殖质组分团聚体的有机碳含量平均值排序为:FA团聚体(8.31 g·kg-1)>HA团聚体(8.25 g·kg-1)>HM团聚体(8.19 g·kg-1);蒙脱石不同腐殖质组分团聚体的有机碳含量平均值排序为:FA团聚体(8.71 g·kg-1)>HA团聚体(8.54 g·kg-1)>HM团聚体(8.47 m2·g-1);高岭石不同腐殖质组分团聚体的总比表面积排序为:FA团聚体(164.54 m2·g-1)>HA团聚体(102.88 m2·g-1)>HM团聚体(90.04 m2·g-1);蒙脱石不同腐殖质组分团聚体的总比表面积排序为:FA团聚体(1012.27 m2·g-1)>HA团聚体(871.63 m2·g-1)>HM团聚体(805.67 m2·g-1);高岭石不同腐殖质组分团聚体的内比表面积排序为:FA团聚体(78.07 m2·g-1)>HA团聚体(42.33 m2·g-1)>HM团聚体(20.51 m2·g-1);蒙脱石不同腐殖质组分团聚体的内比表面积排序为:FA团聚体(843.54 m2·g-1)>HA团聚体(523.31 m2·g-1)>HM团聚体(477.49 m2·g-1).
| 表 2 供试团聚体的理化性质 Table 2 Physical-chemical properties in tested aggregates |
HA比表面积约为2000 m2·g-1, 比黏土矿物和金属氧化物的比表面积大(傅平青等, 2004).黏土矿物与腐殖质胶结形成有机-矿质复合体后, 比表面积显著增加.土壤比表面积与有机碳含量呈正相关(Kaiser et al., 2010).本文以比表面积增大系数, 即团聚体比表面积与单质黏土矿物比表面积的比值来评价不同腐殖质组分对团聚体比表面积的影响.从表 2可以看出, 与单质黏土矿物相比较, 高岭石不同腐殖质组分(FA、HA、HM)团聚体总比表面积增大系数平均值分别为4.64、2.90、2.54;蒙脱石不同腐殖质组分(FA、HA、HM)团聚体总比表面积增大系数平均值分别为2.91、2.50、2.31;高岭石不同腐殖质组分(FA、HA、HM)团聚体内比表面积增大系数平均值分别为9.61、5.21、2.52;蒙脱石不同腐殖质组分(FA、HA、HM)团聚体内比表面积增大系数平均值分别为4.01、2.49、2.27.可见腐殖质FA、HA和HM对团聚体比表面积增加起重要作用, 特别是对团聚体内比表面积增加作用更加明显.3种不同腐殖质组分比较, 对团聚体比表面积增加作用, 特别是对内比表面积增加作用排序为:FA团聚体>HA团聚体>HM团聚体.
3.2 不同腐殖质组分团聚体对苯酚的封存特征采用Freundlich吸附/解吸方程(式(3)、(5))和Langmuir吸附/解吸方程(式(4)、(6)), 对不同腐殖质组分团聚体对苯酚的吸附/解吸等温线进行拟合, 拟合参数见表 3、4.结果表明, 不同腐殖质组分团聚体对苯酚的吸附/解吸行为均符合Freundlich吸附/解吸方程(R2在0.991~0.997之间)和Langmuir吸附/解吸方程(R2在0.993~0.999之间).不同腐殖质组分团聚体对苯酚的吸附/解吸行为可以用Freundlich吸附/解吸方程和Langmuir吸附/解吸方程拟合.应用式(7)和(8)计算得出的不同腐殖质组分团聚体的苯酚封存特征参数结果见表 3、4.表 3结果表明, 高岭石不同腐殖质组分团聚体对苯酚的封存量(Qmirr)排序为:FA团聚体((133.71±6.14) mg·kg-1)>HA团聚体((49.59±8.93) mg·kg-1)>HM团聚体((21.68±2.95) mg·kg-1);封存系数(SR)排序为:FA团聚体(0.53±0.04)>HA团聚体(0.27±0.05)>HM团聚体(0.18±0.03);解吸分配系数(kd)排序为:FA团聚体(47.22±3.14)>HA团聚体(29.68±3.85)>HM团聚体(25.59±3.45).表 4结果表明, 蒙脱石不同腐殖质组分团聚体对苯酚的封存量(Qmirr)排序为:FA团聚体((319.44±8.95) mg·kg-1)>HA团聚体((92.96±22.10) mg·kg-1)>HM团聚体((36.22±6.36) mg·kg-1);封存系数(SR)排序为:FA团聚体(0.76±0.02)> HA(0.32±0.09)>HM团聚体(0.23±0.05);解吸分配系数(ka)排序为:FA团聚体(55.88±4.39)>HA团聚体(40.44±0.72)>HM团聚体(31.42±1.36).
| 表 3 高岭石不同腐殖质组分团聚体对苯酚的吸附-解吸等温线拟合参数及封存特征参数 Table 3 Regression parameters and sequestration characteristics parameters for phenol sorption/desorption on kaolinite aggregates with different humus fraction |
| 表 4 蒙脱石不同腐殖质组分团聚体对苯酚的吸附-解吸等温线拟合参数及封存特征参数 Table 4 Regression parameters and sequestration characteristics parameters for phenol sorption/desorption on montmorillonite aggregates with different humus fraction |
团聚体对苯酚的封存特征参数与团聚体理化性质相关分析结果见表 5.从表 5可以看出, 团聚体对苯酚的吸附分配系数与团聚体有机碳含量、内比表面积、总比表面积呈显著正相关(p < 0.01);团聚体对苯酚的解吸分配系数与团聚体有机碳含量、内比表面积呈显著正相关(p < 0.01), 与总比表面积呈显著正相关(p < 0.05).团聚体对苯酚封存量与团聚体有机碳含量、内比表面积呈显著正相关(p < 0.01), 与总比表面积成显著正相关(p < 0.05);团聚体对苯酚封存系数与团聚体有机碳含量、内比表面积呈显著正相关(p < 0.01), 与总比表面积相关性不显著.3种不同腐殖质组分团聚体对苯酚的封存特征参数(封存量、封存系数)与有机碳含量、比表面积增加系数特别是内比表面积增加系数(表 1)具有高度一致性:FA团聚体>HA团聚体>HM团聚体.说明腐殖质团聚体对苯酚的封存能力的影响是通过增加黏土矿物的腐殖质含量和内比表面积来实现的.
| 表 5 团聚体苯酚封存特征参数与团聚体理化性质相关分析 Table 5 Correlation coefficients ofQmirr、SRwith micro-aggregates physical-chemical properties |
吸附(富集)是封存发生的前提, 天然固体(土壤/沉积物有机-矿质复合体)中具有微孔结构(Li et al., 2001), 研究发现, 有机质可使土壤/沉积物形成复杂孔隙结构(徐红霞等, 2008), 疏水性有机污染物常常被吸附在土壤/沉积物有机-矿质复合体的微孔结构内(Hundal et al., 2001; Li et al., 2004; Ran et al., 2004).苯酚在有机修饰土上的吸附就存在着在土壤孔隙内的吸附作用(孟昭福等, 2009).在固体-水界面上, 有机质的物理构象在调控疏水性有机物(HOC)吸附方面具有重要作用(Feng et al., 2006; Luo et al., 2008), HOC吸附容量取决于有机质的物理构象(Feng et al., 2006), 而凝聚的有机质具有更大的吸附能力(Luo et al., 2008).土壤有机质(SOM)的芳香族和碳水化合物组分分别在有机-矿质复合体(OMC)凝聚和膨胀域的构象中具有重要作用, 并对OMC的吸附行为产生重要的影响(Gunasekara et al., 2003), 而非线性吸附仅在SOM的凝聚域中发生, 在凝聚域中的吸附可能是导致缓慢解吸的原因(Xing, 2001).天然有机质不同组分在平均分子量及化学组成和结构上不同(Weerd et al., 1999), 较小的土壤腐殖酸组分(FA和HA)比较大的组分含有更多的官能团和更大比例的芳族碳(Christl et al., 2000; Watanabe et al., 2006).HM组分以脂肪族结构为主, 烷基碳含量较高, 未饱和的芳香碳和羧基碳含量相对较少(Poirier et al., 2000; Seunghun et al., 2003; Tsutsuki et al., 2012), 溶解性有机质中的芳香族、羧酸类和酚类优先被吸收(Oren et al., 2012).因为这些组分可以更容易地进入层状双氢氧化物介孔, 并且包含更多的羧酸基团(Vreysen et al., 2008).平均分子量分布方面, FA显著低于HA;比表面积和孔容分析表明, 粒径较大的HA和HM易在矿物外表面堆积, 粒径较小的FA比HA和HM更易填塞矿物孔隙(Zhang et al., 2012), 形成微孔结构.微孔似乎有利于形成每个分子的多个复合键(Kaiser et al., 2007), 有机溶质对孔的亲和力比溶解位点更大(Xia et al., 2001).菲和芘进入颗粒内部的径向扩散比基于单个吸附域和扩散率的扩散模型预测的更快(Ahn et al., 2005).吸附在矿物表面的腐殖质形成高度多孔的三维共聚结构对土壤颗粒-溶液界面的反应性具有重要意义(Maurice et al., 1999).与HA和HM团聚体比较, 苯酚更容易吸附在FA团聚体的微孔结构内.表 3结果表明, 高岭石不同腐殖质组分团聚体对苯酚的饱和吸附量(Qm)排序为:FA团聚体((254.11±5.35) mg·kg-1)>HA团聚体((186.14±1.61) mg·kg-1)>HM团聚体((120.61±1.67) mg·kg-1).表 4结果表明, 蒙脱石不同腐殖质组分团聚体对苯酚的Qm排序为:FA团聚体((418.72±19.14) mg·kg-1)>HA团聚体((290.00±13.06) mg·kg-1)>HM团聚体((160.46±4.92) mg·kg-1).
4.2 苯酚封存机理探讨不可逆孔隙变形是造成自然有机物材料中解吸滞后现象的一种机制, 吸附质诱导吸附剂有机基质内部微孔结构在吸附时发生不可逆转的形变, 从而导致吸附和解吸途径的不同(Braida et al., 2003; Sander et al., 2005; 2006).在多孔土壤基质中, 物理包裹(或物理约束)可能是控制解吸阻力的主要机制(Duan, 2008; Yang et al., 2009).针对苯酚吸附机理的研究结果表明, 团聚体对苯酚的吸附以孔隙填充方式为主, 苯酚的Qm排序为:FA团聚体>HA团聚体>HM团聚体.因此, 不同腐殖质组分团聚体对苯酚的封存量(Qmirr)和封存系数(SR)排序为:FA团聚体>HA团聚体>HM团聚体.
2种黏土矿物比较而言, 高岭石是一种不膨胀的1:1层状硅酸盐矿物, 蒙脱石是一种膨胀的2:1层状硅酸盐矿物, 它具有较高的阳离子交换量、溶胀性能和表面积(Ghosh et al., 2009).黏土矿物的化学性质和孔结构通常影响其吸附能力(Ismadji, 2015), 导致它们对FA/HA的亲和力不同, 与高岭石比较, 蒙脱石对HA的吸附亲和力更大(Zhang et al., 2012).土壤对土壤有机质(SOM)的吸附亲和力越高, SOM的凝聚程度越高(Wang et al., 2005), 而凝聚的有机质具有更大的吸附能力(Luo et al., 2008).3种腐殖质组分(FA、HA、HM), 2种黏土矿物(高岭石、蒙脱石)团聚体的苯酚饱和吸附量(Qm, mg·kg-1)分别为:FA(254.11±5.35 < 418.72±19.14)、HA(186.14±1.61 < 290.00±13.06)、HM(120.61±1.67 < 160.46±4.92);苯酚封存系数(SR)分别为:FA(0.53±0.04 < 0.76±0.02)、HA(0.27±0.05 < 0.32±0.09)、HM(0.18±0.03 < 0.23±0.05).2种黏土矿物(高岭石、蒙脱石)的3种腐殖质组分(FA、HA、HM)团聚体的苯酚饱和吸附量和封存系数都表现为高岭石团聚体小于蒙脱石团聚体.
碳质土壤改良剂用于污染土壤和沉积物修复时, 会强力吸附疏水性有机污染物并降低其溶解度, 这就限制了其生物吸收和毒性(Marchal et al., 2013).有机污染物(多环芳烃)经过525 d即可老化, 有机溶剂的萃取率显著降低, 各种有机化合物表观损失的半衰期为96~1789 d, 表明有机污染物(多环芳烃)的封存最长可达10年(Northcott et al., 2001).土壤的有机碳含量、CEC和其他性质与有机化合物的封存有关(Chung et al., 2002).本研究得出, 考查腐殖质团聚体对苯酚的封存能力时不但要考虑腐殖质的含量, 更要考虑腐殖质的赋存形态, 它也是影响团聚体对苯酚封存能力的重要因素.高岭石FA团聚体对苯酚的封存量可达饱和吸附量的50%以上, 蒙脱石FA团聚体对苯酚的封存量可达到饱和吸附量的76%, 因此, FA团聚体可作为治理受苯酚污染场地土壤的一种首选修复剂.
5 结论(Conclusions)1) 不同腐殖质组分团聚体对苯酚的吸附/解吸行为可用Freundlich吸附/解吸方程(R2=0.991~0.998)和Langmuir吸附/解吸方程(R2=0.993~0.999)描述.
2) 不同腐殖质组分黏土矿物团聚体对苯酚的封存量(Qmirr)排序为:FA团聚体>HA团聚体>HM团聚体;不同腐殖质组分黏土矿物团聚体对苯酚的封存系数(SR)排序为:FA团聚体>HA团聚体>HM团聚体.
3) 团聚体对苯酚的吸附/解吸分配系数与团聚体有机碳含量和内比表面积呈显著正相关(p < 0.01), 团聚体对苯酚的封存系数与团聚体有机碳含量和内比表面积呈显著正相关(p < 0.01).
4) 考查腐殖质团聚体对苯酚的封存能力时不但要考虑腐殖质的含量, 更要考虑腐殖质的赋存形态, 它也是影响团聚体对苯酚封存能力的重要因素.不同腐殖质组分团聚体对苯酚封存能力排序为:FA团聚体>HA团聚体>HM团聚体.因此, FA团聚体可作为治理受苯酚污染场地土壤的一种首选修复剂.
Ahn S, Werner D, Karapanagioti H K, et al. 2005. Phenanthrene and pyrene sorption and intraparticle diffusion in polyoxymethylene, coke, and activated carbon[J]. Environmental Science & Technology, 9(17): 6516–6526.
|
Beckles D M, Chen W, Hughes J B. 2007. Bioavailability of polycyclic aromatic hydrocarbons sequestered in sediment:Microbial study and model prediction[J]. Environmental Toxicology & Chemistry, 26(5): 878–883.
|
Braida W J, Pignatello J J, Lu Y, et al. 2003. Sorption hysteresis of benzene in charcoal particles[J]. Environmental Science & Technology, 37(2): 409–417.
|
Carvalho M N, Da M M, Benachour M, et al. 2012. Evaluation of BTEX and phenol removal from aqueous solution by multi-solute adsorption onto smectite organoclay[J]. Journal of Hazardous Materials, 239-240(18): 95–101.
|
Chen J, Wei E, Xian Q. 2014. Pollution status of phenolic compounds in the soil and sediment from a chemical industrial park along the Yangtze River[J]. Se Pu, 32(8): 843–848.
|
Christl I, Knicker H, Kögel-knabner I, et al. 2000. Chemical heterogeneity of humic substances:characterization of size fractions obtained by hollow-fibre ultrafiltration[J]. European Journal of Soil Science, 51(4): 617–625.
DOI:10.1111/ejs.2000.51.issue-4
|
Chung N, Alexander M. 2002. Effect of soil properties on bioavailability and extractability of phenanthrene and atrazine sequestered in soil[J]. Chemosphere, 48(1): 109–115.
DOI:10.1016/S0045-6535(02)00045-0
|
Duchaufour P. 1998. New findings on humification in forest soils under temperate conditions[J]. Eurasian Soil Science, 31(7): 803–808.
|
Duan L, Zhang N, Wang Y, et al. 2008. Release of hexachlorocyclohexanes from historically and freshly contaminated soils in China:implications for fate and regulation[J]. Environmental Pollution, 156(3): 753–759.
DOI:10.1016/j.envpol.2008.06.006
|
Feng X J, Simpson A J, Simpson M J. 2006. .Investigating the role of mineral-bound humic acid in phenanthrenesorption[J]. Environmental Science & Technology, 40(10): 3260–3266.
|
傅平青, 刘丛强, 吴丰昌. 2004. 水环境中腐殖质-金属离子键合作用研究进展[J]. 生态学杂志, 2004, 23(6): 143–148.
DOI:10.3321/j.issn:1000-4890.2004.06.030 |
Gondar D, Lopez R, Fiol S, et al. 2005. Characterization and acid-base properties of fulvic and humic acids isolated from two horizons of an ombrotrophic peat bog[J]. Geoderma, 126(3/4): 367–374.
|
Ghosh U, Gillette J S, Luthy R G, et al. 2000. Microscale location, characterization, and association of polycyclicaromatic hydrocarbons on harbor sediment particles[J]. Environmental Science & Technology, 34(9): 1729–1736.
|
Ghosh S, Wang Z Y, Kang S, et al. 2009. Sorption and fractionation of a peat derived humic acid by kaolinite, montmorillonite, and goethite[J]. Pedosphere, 19(1): 21–30.
DOI:10.1016/S1002-0160(08)60080-6
|
Gunasekara A S, Simpson M I, Xing B. 2003. Identification and characterization of sorption domains in soil organic matter using strucuturally modified humic acids[J]. Environmental Science & Technology, 37(5): 852–858.
|
Han L, Sun K, Jin J, et al. 2016. Some concepts of soil organic carbon characteristics and mineral interaction from a review of literature[J]. Soil Biology & Biochemistry, 94(3): 107–121.
|
贺婧, 刘田, 关连珠. 2012. 碳酸钙对土壤吸附苯酚特性的影响[J]. 沈阳农业大学学报, 2012, 43(3): 362–365.
DOI:10.3969/j.issn.1000-1700.2012.03.020 |
Huang L, Zhou Y, Guo X, et al. 2015. Simultaneous removal of 2, 4-dichlorophenol and Pb(Ⅱ) from aqueous solution using organoclays:Isotherm, kinetics and mechanism[J]. Journal of Industrial & Engineering Chemistry, 22(2): 280–287.
|
Huang W, Schlautman M A, Weber W J. 1996. A distributed reactivity model for sorption by soils and sediments.5.the influence of near-surface characteristics in mineral domains[J]. Environmental Science & Technology, 30(10): 2993–3000.
|
Huang Q, Liang W, Cai P. 2005. Adsorption, desorption and activities of acid phosphatase on various colloidal particles from an Ultisol[J]. Colloids & Surfaces B Biointerfaces, 45(3/4): 209–214.
|
Hundal L S, Thompson M L, Laird D A, et al. 2001. Sorption of phenanthrene by reference smectites[J]. Environmental Science & Technology, 35(17): 3456–3461.
|
Ismadji S, Soetaredjo F E, Ayucitra A. 2015. Modification of clay minerals for adsorption purpose[J]. Springer International Publishing.
DOI:10.1007/978-3-319-16712-1_3
|
Jackson D R, Bisson D L. 1990. Mobility of polychlorinated aromatic compounds in soils contaminated with wood-preserving oil[J]. Journal of the Air & Waste management Association, 40(8): 1129–1133.
|
Kan A T, Fu G, Hunter M A, et al. 1997. Irreversible adsorption of naphthalene and tetrachlorobiphenyl to Lula and Surrogate Sediments[J]. Environmental Science & Technology, 31(8): 2176–2185.
|
Kan A T, Fu G, Hunter M, et al. 1998. Irreversible sorption of neutral hydrocarbons to sediments:experimental observations and model predictions[J]. Environmental Science & Technology, 32(7): 892–902.
|
Ksiezopolska A, Pazur M. 2011. Surface properties of bentonite and illite complexes with humus acids[J]. Clay Minerals, 46(1): 149–156.
DOI:10.1180/claymin.2011.046.1.149
|
Kögel-knabner I, Guggenberger G, Kleber M, et al. 2008. Organo-mineral associations in temperate soils:Integrating biology, mineralogy, and organic matter chemistry[J]. Journal of Plant Nutrition & Soil Science, 171(1): 61–82.
|
Kalbitz K, Schwesig D, Rethemeyer J, et al. 2005. Stabilization of dissolved organic matter by sorption to the mineral soil[J]. Soil Biology and Biochemistry, 37(7): 1319–1331.
DOI:10.1016/j.soilbio.2004.11.028
|
Kaiser K, Guggenberger G. 2000. The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils[J]. Organic Geochemistry, 31(7/8): 711–725.
|
Kaiser K, Guggenberger G. 2010. Mineral surfaces and soil organic matter[J]. European Journal of Soil Science, 54(2): 219–236.
|
Kaiser K, Guggenberger G. 2007. Sorptive stabilization of organic matter by microporous goethite:sorption into small pores vs. surface complexation[J]. European Journal of Soil Science, 58(1): 45–59.
DOI:10.1111/ejs.2007.58.issue-1
|
Kang S H, Xing B S. 2008. Humic acid fractionation upon sequential adsorption onto goethite[J]. Langmuir the Acs Journal of Surfaces & Colloids, 24(6): 2525–2531.
|
Khairy M A. 2013. Assessment of priority phenolic compounds in sediments from an extremely polluted coastal wetland (Lake Maryut, Egypt)[J]. Environmental Monitoring & Assessment, 185(1): 441–455.
|
Lamar R T, White R B, Ashley K C. 2010. Evaluation of white-rot fungi for the remediation of creosote-contaminated soil[J]. Remediation Journal, 12(4): 97–106.
|
Lehtonen K, Hänninen K, Ketola M. 2001. Structurally bound lipids in peat humic acids[J]. Organic Geochemistry, 32(1): 33–43.
DOI:10.1016/S0146-6380(00)00158-3
|
Litvin V A, Galagan R L, Minaev B F. 2012. Synthesis and properties of synthetic analogs of natural humic acids[J]. Russian Journal of Applied Chemistry, 85(2): 296–302.
DOI:10.1134/S1070427212020243
|
Li J, Werth C J. 2001. Evaluating competitive sorption mechanisms of volatile organic compounds in soils and sediments using polymers and zeolites[J]. Environmental Science & Technology, 35(3): 568–574.
|
Li J, Werth C J. 2004. Slow desorption mechanisms of volatile organic chemical mixtures in soil and sediment micropores[J]. Environmental Science & Technology, 38(2): 440–448.
|
鲁如坤. 2000. 土壤农业化学分析方法[M]. 北京: 中国农业科技出版社: 39–110.
|
Luo L, Zhang S, Ma Y, et al. 2008. Facilitating effects of metal cations on phenanthrone sorption in soils[J]. Environmental Science & Technology, 42(7): 2414–2419.
|
Luthy R G, Aiken G R, Brusseau M L, et al. 1997. Sequestration of hydrophobic organic contaminants by geosorbents[J]. Environmental Science & Technology, 31(12): 3341–3347.
|
Maurice P A, Namjesnik-Dejanovic K. 1999. Aggregate structures of sorbed humic substances observed in aqueous solution[J]. Environmental Science and Technology, 33(9): 1538–1541.
DOI:10.1021/es981113+
|
Ma L, Chen Q, Zhu J, et al. 2016. Adsorption of phenol and Cu(Ⅱ) onto cationic and zwitterionic surfactant modified montmorillonite in single and binary systems[J]. Chemical Engineering Journal, 283: 880–888.
DOI:10.1016/j.cej.2015.08.009
|
孟昭福, 邓晶, 扬淑英, 等. 2009. 有机修饰土娄土对苯酚的吸附动力学[J]. 环境科学, 2009, 30(1): 191–199.
|
Mader B T, Uwe-Goss K, Eisenreich S J. 1997. Sorption of nonionic, hydrophobic organic chemicals to mineral surfaces[J]. Environmental Science & Technology, 31(4): 1079–1086.
|
Marchal G, Smith K E, Rein A, et al. 2013. Comparing the desorption and biodegradation of low concentrations of phenanthrene sorbed to activated carbon, biochar and compost[J]. Chemosphere, 90(6): 1767–1778.
DOI:10.1016/j.chemosphere.2012.07.048
|
Murphy E M, Zachara J M, Smith S C, et al. 1994. Interaction of hydrophobic organic compounds with mineral-bound humic substances[J]. Environmental Science & Technology, 28(7): 1291–1299.
|
Murphy E M, Zachara J M, Smith S C. 1990. Influence of mineral-bound humic substances on the sorption of hydrophobic organic compounds[J]. Environmental Science & Technology, 24(10): 1507–1516.
|
倪进治, 骆永明, 魏然, 等. 2008. 不同离子桥键的有机矿质复合体对菲的吸附-解吸研究[J]. 环境科学, 2008, 29(12): 3531–3536.
DOI:10.3321/j.issn:0250-3301.2008.12.040 |
Northcott G L, Jones K C. 2001. Partioning, extractability, and formation of nonextractable PAH residues in soil.1.Compound differences in aging and sequestration[J]. Environmental Science & Technology, 35(6): 1103–1110.
|
Oades J M. 1988. The retention of organic matter in soils[J]. Biogeochemistry, 5(1): 35–70.
DOI:10.1007/BF02180317
|
Oren A, Chefetz B. 2012. Sorptive and desorptive fractionation of dissolved organic matter by mineral soil matrices[J]. Journal of Environmental Quality, 41(2): 526–533.
DOI:10.2134/jeq2011.0362
|
Pan B, Tao S, Dawson R W, et al. 2010. Formation of organo-mineral complexes as affected by particle size, pH, and dry-wet cycles[J]. Soil Research, 48(8): 713–719.
DOI:10.1071/SR10029
|
Park Y, Ayoko G A, Kurdi R, et al. 2013. Adsorption of phenolic compounds by organoclays:implications for the removal of organic pollutants from aqueous media[J]. Journal of Colloid & Interface Science, 406(18): 196–208.
|
裴芳, 罗泽娇, 彭进进, 等. 2012. 某炼油厂退役场地土壤与浅层地下水酚类污染特征研究[J]. 环境科学, 2012, 33(12): 4251–4255.
|
Poirier N, Derenne S, Rouzaud J N, et al. 2000. Chemical structure and sources of the macromolecular, resistant, organic fraction isolated from a forest soil (Lacadée, south-west France)[J]. Organic Geochemistry, 31(9): 813–827.
DOI:10.1016/S0146-6380(00)00067-X
|
Ran Y, Xing B, Suresh P, et al. 2004. Importance of adsorption (hole-filling) mechanism for hydrophobic organic contaminants on an aquifer kerogen isolate[J]. Environmental Science & Technology, 38(16): 4340–4348.
|
任爽, 孟昭福, 王腾, 等. 2017. 阳阴离子复配修饰两性磁性膨润土的表面特征差异及对苯酚的吸附的影响[J]. 环境科学, 2017, 36(8): 187–194.
|
Sander M, Pignatello J J. 2005. An isotope exchange technique to assess mechanisms of sorption hysteresis applied to naphthalene in kerogenous organic matter[J]. Environmental Science & Technology, 39(19): 7476–7484.
|
Sander M, Lu Y, Pignatello J J. 2006. Conditioning-annealing studies of natural organic matter solids linking irreversible sorption to irreversible structural expansion[J]. Environmental Science & Technology, 40(1): 170–178.
|
Shaker A M, Komy Z R, Heggy S E, et al. 2012. Kinetic study for adsorption humic acid on soil minerals[J]. Journal of Physical Chemistry A, 116(45): 10889–10896.
DOI:10.1021/jp3078826
|
Shang J, Chen J, Shen Z, et al. 2013. Effects of varying estuarine conditions on the sorption of phenanthrene to sediment particles of Yangtze Estuary[J]. Marine Pollution Bulletin, 76(1/2): 139–145.
|
Schaumann G E, Bertmer M. 2010. Do water molecules bridge soil organic matter molecule segments[J]. European Journal of Soil Science, 59(3): 423–429.
|
Shirshova L T, Ghabbour E A, Davies G. 2006. Spectroscopic characterization of humic acid fractions isolated from soil using different extraction procedures[J]. Geoderma, 133(3/4): 204–216.
|
Seunghun K, Dula A, Peter V, et al. 2003. Characterization of ten sequentially extracted humic acids and a humin from a soil in western massachusetts[J]. Soil Science, 168(12): 880–887.
DOI:10.1097/01.ss.0000106404.84926.b0
|
Terashima M, Tanaka S, Fukushima M. 2003. Distribution behavior of pyrene to adsorbed humic acids on kaolin[J]. Journal of Environmental Quality, 32(2): 591–598.
DOI:10.2134/jeq2003.5910
|
Tomson M B. 2006. Sequestration of organic contaminants in soil/sediment and its impact on contaminant fate[J]. Acta Geochimica, 25(s1): 262–263.
|
Tombácz E, Libor Z, Illés E, et al. 2004. The role of reactive surface sites and complexation by humic acids in the interaction of clay mineral and iron oxide particles[J]. Organic Geochemistry, 35(3): 257–267.
DOI:10.1016/j.orggeochem.2003.11.002
|
Tsutsuki K, Kuwatsuka S. 2012. Characterization of humin-metal complexes in a buriedvolcanic ash soil profile and apeat soil[J]. Soil Science and Plant Nutrition, 38(2): 297–306.
|
Vreysen S, Maes A. 2008. Adsorption mechanism of humic and fulvic acid onto Mg/Al layered double hydroxides[J]. Applied Clay Science, 38(3/4): 237–249.
|
Wang K, Xing B. 2005. Structural and sorption characteristics of adsorbed humic acid on clay minerals[J]. Journal of Environmental Quality, 34(1): 342–349.
DOI:10.2134/jeq2005.0342
|
Wang X, Sato A T, Xing B. 2005. Sorption and displacement of pyrene in soils and sediments[J]. Environmental Science & Technology, 39(22): 8712–8718.
|
Wang Y, Xu J, Shan J, et al. 2017. Fate of phenanthrene and mineralization of its non-extractable residues in an oxic soil[J]. Environmental Pollution, 224: 377.
|
Watanabe A, Kuwatsuka S. 2006. Chemical characteristics of soil fulvic acids fractionated using polyvinylpyrrolidone (PVP)[J]. Soil Science & Plant Nutrition, 38(1): 31–41.
|
Wei S Y, Tan W F, Liu F, et al. 2014. Surface properties and phosphate adsorption of binary systems containing goethite and kaolinite[J]. Geoderma, 213(1): 478–484.
|
Weerd H V D, Riemsdijk W H V, Leijnse A. 1999. Modeling the dynamic adsorption/desorption of a NOM mixture:effects of physical and chemical heterogeneity[J]. Environ Sci Technol, 33(10): 1675–1681.
DOI:10.1021/es980815w
|
Xia G, Pignatello J J. 2001. Detailed sorption isotherms of polar and apolar compounds in a high-organic soil[J]. Environmental Science & Technology, 35(1): 84–94.
|
Xing B. 2001. Sorption of naphthalene and phenanthrene by soil humic acids[J]. Environmental Pollution, 111(2): 303–309.
DOI:10.1016/S0269-7491(00)00065-8
|
徐红霞, 吴海燕, 张景飞, 等. 2008. 2, 4-二氯苯酚在太湖沉积物中的吸附/解吸行为研究[J]. 农业环境科学学报, 2008, 27(4): 1415–1420.
DOI:10.3321/j.issn:1672-2043.2008.04.023 |
Yang Y, Ratté D, Smets B F, et al. 2001. Mobilization of soil organic matter by complexing agents and implications for polycyclic aromatic hydrocarbon desorption[J]. Chemosphere, 43(8): 1013–1021.
DOI:10.1016/S0045-6535(00)00498-7
|
Yang W C, Zhang J, Zhang C D, et al. 2009. Sorption and resistant desorption of atrazine in typical Chinese soils[J]. Journal of Environmental Quality, 38(1): 171–178.
DOI:10.2134/jeq2007.0674
|
尹勇, 戴中华, 蒋鹏, 等. 2012. 苏南某焦化厂场地土壤和地下水特征污染物分布规律研究[J]. 农业环境科学学报, 2012, 31(8): 1525–1531.
|
Zhang L, Luo L, Zhang S. 2011. Adsorption of phenanthrene and 1, 3-dinitrobenzene on cation-modified clay minerals[J]. Colloids & Surfaces A Physicochemical & Engine, 377(1): 278–283.
|
Zhang L, Luo L, Zhang S. 2012. Integrated investigations on the adsorption mechanisms of fulvic and humic acids on three clay minerals[J]. Colloids & Surfaces A Physicochemical & Engine, 406(1): 84–90.
|
Zhou M, Zhang J, Sun C. 2017. Occurrence, ecological and human health risks, and seasonal variations of phenolic compounds in surface water and sediment of a potential polluted River Ba[J]. [J].International Journal of Environmental Research, 14(10): 1140.
|
Zhu X, He J, Su S, et al. 2016. Concept model of the formation process of humic acid-kaolin complexes deduced by trichloroethylene sorption experiments and various characterizations[J]. Chemosphere, 151(10): 116–123.
|
 2019, Vol. 39
2019, Vol. 39


