2. 同济大学环境科学与工程学院, 长江水环境教育部重点实验室, 上海 200092;
3. 瑞典自然历史博物馆, 斯德哥尔摩 SE-10405;
4. 斯德哥尔摩大学环境科学与分析化学系, 斯德哥尔摩 SE-10691
2. Key Laboratory of Yangtze River Water Environment of the Ministry of Education, College of Environmental Science and Engineering, Tongji University, Shanghai 200092;
3. Swedish Natural History Museum, Stockholm SE-10405;
4. Department of Environmental Science and Analytical Chemistry(ACES), Stockholm University, Stockholm SE-10691
有机氯农药(OCPs)曾因大量生产和广泛使用而释放到环境中, 多数具有持久性、生物累积性和毒性, 曾在全世界范围内对鱼类、水生哺乳类和鸟类等野生动物及人类健康造成风险(Focardi et al., 1996), 因而被列入持久性有机污染物(POPs)清单之中.某些OCPs及其代谢物具有内分泌干扰作用, 如滴滴伊(DDE)作为滴滴涕(DDT)的稳定代谢物, 可持久地干扰鸟类的钙磷代谢, 导致卵壳变薄, 进而影响种群繁殖(Bignert et al., 2015).有机氯化学品等POPs可能在低营养级生物中无法检出, 但在较高营养级鸟类的卵、肌肉、肝脏和羽毛等组织及其猎物中可以找到(Albanis et al., 1996;Padula et al., 2010;陈静等, 2013;Huertas et al., 2016;Ye et al., 2016), 因此, 鸟卵可以有效地用于筛查残留POPs和新兴污染物的存在, 监测其暴露和效应的变化趋势等(Chen et al., 2012;Bignert et al., 2015;Greaves et al., 2016;Champoux et al., 2017;Schmitt et al., 2018), 具有重要的研究价值.
欧美国家较早利用鸟卵从事相关研究, 试图确认有毒化学品污染与其种群数量减少之间的联系.以夜鹭(也称黑冠夜鹭, 拉丁名Nycticorax nycticorax)为例, 曾在美国西部山区的繁殖力较低, 后来发现可能与夜鹭卵中p, p′-DDE含量过高导致蛋壳变薄有关(Henny et al., 1984).当然, 随着夜鹭卵中DDE和多氯联苯(PCBs)残留量的降低, 蛋壳厚度可能回升(Ohlendorf et al., 1978;Custer et al., 1983).尽管水禽样品(尤其是夜鹭卵)中OCPs含量在近20年呈降低趋势, 但DDE和PCBs等依然在某些样点会表现亚致死效应和影响繁殖(Mora et al., 2016; Rattner et al., 2007);研究发现, 夜鹭雏鸟体内存在OCPs及其代谢物的积累(Rattner et al., 1997).我国对水鸟中OCPs的研究始于10多年前, 主要集中在珠三角和长三角等发达地区, 也发现鹭卵中存在较高的p, p′-DDE残留(董元华等, 2002;安琼等, 2004;Wang et al., 2011).另外, 还在鹭卵中检测到许多痕量元素、POPs和一些新兴污染物(龚钟明等, 2001;Lam et al., 2004;Lam et al., 2008;Luo et al., 2009;Hu et al., 2016;Zhou et al., 2016a; 2016b).然而, 关于长江上游地区鹭科鸟类样品中POPs的赋存状况及其在鹭卵孵化过程中的变化还缺乏了解.
宜宾地处长江上游地区四川盆地边缘, 素有“长江第一城”之称, 其地貌特征以中低山地和丘陵为主体, 岭谷相间, 拥有丰富多样的鸟类生境和食物来源, 吸引了大量的鸟类在此栖息繁衍, 而鸟类摄食和排泄活动可能会分别给当地渔业资源和环境质量带来影响(黄强等, 1995;廖峻涛等, 2013;李雷等, 2013).本文拟以夜鹭为例, 分析鹭卵中有机氯农药及其代谢物的赋存特征, 试图了解鹭卵中残留的OCPs对其繁殖力的潜在影响, 探究鹭卵孵化过程对不同OCPs残留特征的影响.
2 材料与方法(Material and methods) 2.1 样品采集宜宾境内鸟类资源丰富, 不同生境的鸟类有255种及22亚种(黄强等, 1995).鹭科鸟类种类和数量均较多, 其中, 黑冠夜鹭(N. nycticorax)大部分为夏候鸟, 通常于3月中下旬陆续到此繁殖(孵卵期22~26 d), 常成群在一起营群巢于高大树干上, 主要以鱼、蛙、虾、水生昆虫等动物为食(Fasola et al., 1998), 9月末陆续迁离, 部分为留鸟.
2017年5月底—6月初, 多次在长江上游宜宾地区采集夜鹭的卵样品, 此时大部分鸟巢都有鹭鸟守护着新生的雏鸟或还在孵卵, 主要从没有鹭鸟守护的树巢中采集鹭卵(可能是未成功孵化的).这些鸟蛋呈蓝绿色, 随机采集于长宁县桃坪乡什字水库(104.98°E, 28.54°N, 见图 1)周边树林鸟巢中.有研究报道, 夜鹭同一窝卵之间的OCPs含量差异比不同窝卵之间的差异要小很多(Custer et al., 1990), 同时考虑到采样尽可能不要破坏鹭鸟的繁殖活动, 因此, 每个鸟窝中仅取1枚, 一共采集30枚.采集时, 用棉花包裹后放在铝盒中, 带回实验室后临时存放于4 ℃冷藏冰箱中.用清水冲洗蛋壳表面, 擦干表面后进行卵的质量和形态参数测量;经光照检查, 9枚为无胚卵, 21枚为胚胎卵, 将蛋液取出至洁净玻璃瓶(经高温灼烧, 瓶盖用丙酮和正己烷清洗)中并搅拌均匀, 在长江环境样品库(YESB)于-80 ℃下冷冻保存至分析.
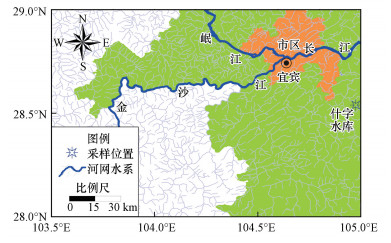 |
| 图 1 鹭卵采样地理位置示意图 Fig. 1 Geographic location of heron egg sampling |
鸟蛋样品中有机氯化合物的分析在芬兰国立卫生和福利研究院进行, 参考小体积血清样品中POPs分析方法(Koponen et al., 2013), 其提取和净化过程具体如下:在200 μL匀浆后的蛋液等份样品中加入250 μL乙醇和400 pg 13C标记的各种OCPs内标物(溶于100 μL甲苯中, 剑桥同位素实验室CIL, 美国), 在多管涡流器(VWR DVX-2500, Henry Troemner, USA)上以1800 r · min-1的速度混合4 min使蛋白质沉淀并平衡内标.用2 mL二氯甲烷/正己烷(DCM/HEX, 1 : 4, V/V)进行提取, 然后加入0.5 mL活性硅胶除去样品中的水分和乙醇.以1800 r · min-1的速度将样品混合4 min, 并在混合后将上部DCM/HEX萃取液倒入8 mL固相萃取柱(SPE柱)中进行净化, OCPs提取过程重复2次.该SPE柱依次填充了1 mL 44%硫酸硅土(底层)、2 mL 10%硝酸银浸渍硅土(中层)和2 mL硫酸钠/硅土混合物(1 : 2, V/V, 上层)等, 使用前用4 mL DCM/HEX(1 : 4)洗涤柱.用4 mL DCM/HEX(1 : 4)从净化柱中洗脱OCPs, 洗脱液用温和的N2浓缩至约0.5 mL.将浓缩的洗脱液转移到自动进样瓶中, 加入100 μL含有400 pg回收率指示物(13C-PCB-128, 美国CIL)的正己烷中, 并加入15~20 μL壬烷作为保存剂.在环境条件下, 将样品蒸发至15~20 μL壬烷的最终体积(以防止灰尘污染), 并在-20 ℃下储存, 直到GC-MS/MS测定.
2.3 仪器分析使用Agilent 7890A气相色谱仪/Agilent 7010三重四极杆质谱仪和DB-5MS UI柱(20 m×0.18 mm I.D.×0.18 μm膜厚, J&W Scientific)通过多重反应监测进行定量.仪器条件参考文献(Koponen et al., 2013), 具体如下:将5 μL净化并浓缩的提取液注入到溶剂排空模式下的程序升温气化(PTV)进样器.排气流量为100 mL · min-1, 排气时间为0.2 min, 排气压力为96.5 kPa.PTV注射器的温度程序为:80 ℃保持0.25 min, 以720 ℃ · min-1升至300 ℃, 保持9.0 min, 再以100 ℃ · min-1升至80 ℃.氦气载气至柱的流速为0.7 mL · min-1.GC烘箱的温度程序为:70 ℃保持2 min, 以60 ℃ · min-1升至190 ℃, 以12.5 ℃ · min-1升至260 ℃, 再以60 ℃ · min-1升至300 ℃, 保持1.2 min.从GC到MS的传输线温度为280 ℃, 电离室温度为270 ℃, 电离能为70 eV.
2.4 质量保证与质量控制实验室测试符合芬兰认证服务, 通过标准参考物多次重复测定和实验室之间比对, 上述POPs测定的准确性和可重复性满足要求(Koponen et al., 2013).欧盟推荐采用定量限(LOQ)和测量不确定度(MU)来评估方法的表现(Lucentini et al., 2013), LOQ通过分析OCPs含量(以湿重计, 下同)尽可能低的5个重复样品获得, 先计算测量值的标准偏差(SD), 再以8倍SD来计算LOQ.如表 1所示, 9种OCPs测量的定量限为5~ 40 pg · g-1, 相对扩展不确定度为分别为30%~55%(< 50 pg · g-1)和20%~50%(>50 pg · g-1).
| 表 1 鹭卵中目标有机氯化合物基本信息及其分析方法有效性 Table 1 Basic information on targeted organochlorine compounds in heron eggs and validation of methods |
如图 2所示, 宜宾夜鹭卵中发现了8种有机氯化合物残留, 而均未检出γ-HCH, 其总含量(∑OCPs)变化范围为4.76 ~ 97.9 ng · g-1(以湿重计, 下同), 个体间变异系数为72%.其中, p, p′-DDE、HCB、β-HCH、PeCB、反式九氯和氧化氯丹几乎在所有的夜鹭卵中存在, 平均含量分别为20.1、5.28、1.10、0.48、0.29和0.17 ng · g-1;α-HCH和p, p′-DDT仅有少量检出(13%~37%), 含量范围分别为ND~0.27 ng · g-1和ND~0.49 ng · g-1.不同鹭卵中OCPs残留水平差异很大(图 2), 可能是个体间同期习惯性取食区域差异造成的, 还可能与产卵顺序及孵化过程等因素有关(Custer et al., 1990; 王培潮, 1992;Hothem et al., 1995;Klein et al., 2012;Bignert et al., 2015).另外, 宜宾的夜鹭卵中p, p′-DDT/p, p′-DDE比值非常小, 小于0.03.显然, 当地的滴滴涕类(DDTs)以p, p′-DDT的代谢物p, p′-DDE为主, 这与四川环境中OCPs以历史残留为主要来源的赋存特征相吻合(邢新丽等, 2009).
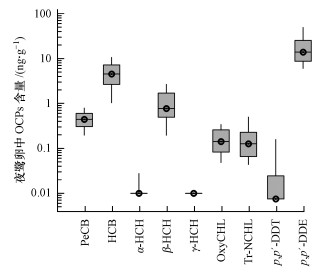 |
| 图 2 夜鹭卵中各种有机氯农药及其代谢物的含量分布箱式图 Fig. 2 Box and whisker plot for the distribution of OCPs concentrations in eggs of black-crowned night heron (Nycticorax nycticorax) |
纵观全球范围内夜鹭卵中各类OCPs的残留水平(表 2, 以湿重计)), 可以发现, 欧美国家的夜鹭卵中∑DDTs残留量在30~40年以前很高(Custer et al., 1983;Fossi et al., 1984;McEwen et al., 1984;Ohlendorf et al., 1990), 高含量的p, p′-DDE甚至会使得蛋壳变薄, 影响夜鹭繁殖(Henny et al., 1984), 而在20世纪90年代末∑DDTs残留水平有了显著降低(Aurigi et al., 2000;Custer et al., 2014).21世纪初我国南方沿海和长三角等发达地区夜鹭卵中∑DDTs的含量也较高, 并出现p, p′-DDE含量超过影响繁殖的效应阈值(1000 ng · g-1)的情形(Connell et al., 2003;Dong et al., 2004;Lam et al., 2008;Wang et al., 2011).长江上游山区夜鹭卵中∑DDTs和∑HCHs的含量均明显低于长三角平原地区, 长江上游和下游山区夜鹭卵中∑DDTs含量没有明显差异, 而在下游山区∑HCHs含量还是显著高于上游山区, 但要显著低于长三角平原地区.而对于氯丹类(∑CHLs)的残留, 长江流域上、下游几个采样点之间没有显著差异, 但均低于我国南方地区和发达国家的残留水平.对于氯苯类(∑CBz)的残留, 我国几个采样点之间没有显著差异, 但要低于欧美国家以前的水平.总体上, 长江上游夜鹭卵中OCPs残留在全球范围和历史上均处于最低水平, 也反映当地OCPs污染相对较低.
| 表 2 国内外夜鹭卵中有机氯农药及其代谢物残留特征的比较 Table 2 Comparison among the residues of four main groups of organochlorine pesticides and metabolites in eggs of black-crowned night heron (Nycticorax nycticorax) from worldwide |
宜宾夜鹭卵的形态差异性较小, 平均长度和宽度分别为44.6 mm和33.6 mm, 变异系数均小于5%, 但这些鹭卵的质量为(18.4±3.9) g, 变异系数达21%.这些鹭卵的形态参数和质量与OCPs残留水平之间并没有显著相关性.大小差不多的夜鹭卵的质量存在较大差异, 可能与孵化过程中鸟卵水分损失和脂类代谢消耗有关(王培潮, 1992;Rattner et al., 2016).因此, 若要比较鸟卵样品之间的OCPs等污染物含量差异, 最好用鸟卵的新鲜湿重进行归一化校正(Rattner et al., 2016).
孵化过程对鹭卵OCPs残留水平的影响较为有限, 如图 3所示, 只有3种OCPs的残留水平在无胚卵样(n=9)的纯蛋液与胚胎卵样(n=21)的蛋液之间存在显著差异, 分别为反式九氯(p < 0.001)、DDT(p < 0.005)、DDE(p < 0.05).孵化过程中, 将消耗一部分蛋液(卵黄和卵白)形成胚胎, 从而导致水分和脂类等物质损失(王培潮, 1992;Rattner et al., 2016).假如卵中OCPs较稳定而没有损失, 剩余蛋液中OCPs含量应该增加或者与胚胎吸收过程有关.然而, 只有反式九氯在胚胎卵蛋液中的残留水平(平均为0.37 ng · g-1)比在无胚卵蛋液中高一些, 而p, p′-DDT和p, p′-DDE在无胚卵蛋液中的残留水平(平均分别为0.07 ng · g-1和20.9 ng · g-1)比在胚胎卵蛋液中更高些, 其余OCPs没有表现出这种差异性.孵化过程中, 卵黄/卵白中DDT的损失可能与其代谢过程有关;而持久性较强的DDE损失, 则可能是向胚胎转移.曾有研究报道, 刚出生1 d的夜鹭幼鸟OCPs残留水平比鹭卵要高或与之持平(Custer et al., 1995).因此, 胚胎形成过程中, 较稳定的OCPs将在胚胎卵内部组织中重新分配, p, p′-DDE可能比反式九氯更容易从蛋液向下一代传递.
 |
| 图 3 夜鹭的无胚卵与胚胎卵蛋液中典型有机氯化合物的含量比较 Fig. 3 Comparison of OCPs concentrations in liquid samples from embryo free eggs and embryogenic eggs of black-crowned night heron (Nycticorax nycticorax) |
由于鸟卵中过高的p, p′-DDE含量将导致蛋壳变薄, 进而影响繁殖.宜宾鹭卵中p, p′-DDE含量为3.31~84.2 ng · g-1, 对∑OCPs贡献最大(70%±14%).但该污染物浓度远低于鹭科的繁殖效应阈值1000 ng · g-1 (Connell et al., 2003; Wang et al., 2011), 显然, 长江上游地区夜鹭卵中DDE的存在水平不足以抑制夜鹭繁殖力或幼鸟存活率.那么, 对于营养层次更高的鹭科鸟类是否会有影响?研究表明, 多瑙河三角洲白鹭卵中DDTs和PCBs等有机氯化学品的含量显著高于夜鹭卵, 主要原因在于前者在食物链中处于更高的层次及其迁徙习性(Aurigi et al., 2000), 我国香港(珠江三角洲)鹭卵中DDTs、PCBs、CHLs等也有类似规律(Connell et al., 2003).另外, 白鹭比夜鹭在行为上更为活跃, 取食行为比例较高, 单位体重消耗量大(张国钢等, 2007), 因而对OCPs的生物积累和放大作用可能会更明显些, 有必要进一步调查宜宾白鹭卵中含量以确认其繁殖效应.
4 结论(Conclusions)1) 长江上游地区夜鹭卵中普遍存在有机氯农药及其代谢物, 但残留水平差异较大, 其中, p, p′-DDE残留浓度最高, 但其在全球范围内和历史上处于较低水平, 暂不足以影响夜鹭的繁殖.
2) 夜鹭可以通过产卵方式和孵化过程将有机氯物质排出体外并转移到下一代中, 而且p, p′-DDE可能比反式九氯更容易从蛋液向下一代传递.
3) 宜宾周边区域有机氯农药污染相对较低, 主要来自历史残留.当然, 需要进一步评估鹭卵中其他POPs的污染及暴露情况, 以正确认识该地区环境污染与鸟类繁殖之间的关系.
致谢: 感谢中瑞国际合作研究项目Chemstrres-YRD协调员Sune Eriksson博士及芬兰国立卫生与福利研究院Rantakokko Panu博士等在样品分析测试方面提供的帮助.
Albanis T A, Hela D, Papakostas G, et al. 1996. Concentration and bioaccumulation of organochlorine pesticide residues in herons and their prey in wetlands of Thermaikos Gulf, Macedonia, Greece[J]. Science of the Total Environment, 182(1/3): 11–19.
|
安琼, 董元华, 王辉, 等. 2004. 不同年龄夜鹭卵中有机氯农药污染的生物指示[J]. 环境科学学报, 2004, 24(1): 139–143.
DOI:10.3321/j.issn:0253-2468.2004.01.027 |
Aurigi S, Focardi S, Hulea D, et al. 2000. Organochlorine contamination in bird's eggs from the Danube Delta[J]. Environmental Pollution, 109(1): 61–67.
|
Bignert A, Helander B O. 2000. Monitoring of contaminants and their effects on the common guillemot and the white-tailed sea eagle[J]. Journal of Ornithology, 156(sp1): 173–185.
|
Champoux L, Boily M. 2017. Temporal trends of mercury and organohalogen contaminants in great blue heron eggs from the St.Lawrence River, Québec, Canada, 1991-2011, and relationships with tracers of feeding ecology[J]. Science of the Total Environment, 609: 1270–1285.
DOI:10.1016/j.scitotenv.2017.07.223
|
Chen D, Letcher R J, Chu S. 2012. Determination of non-halogenated, chlorinated and brominated organophosphate flame retardants in herring gull eggs based on liquid chromatography-tandem quadrupole mass spectrometry[J]. Journal of Chromatography A, 1220(2): 169–174.
|
陈静, 袁林喜, 祁士华, 等. 2013. 洪湖湿地鹭科鸟类组织中有机氯农药的分布特征及风险评价[J]. 环境化学, 2013, 32(4): 549–556.
|
Connell D W, Fung C N, Minh T B, et al. 2003. Risk to breeding success of fish-eating ardeids due to persistent organic contaminants in Hong Kong:evidence from organochlorine compounds in eggs[J]. Water Research, 37(2): 459–467.
DOI:10.1016/S0043-1354(02)00294-4
|
Custer T W, Custer C M. 1995. Transfer and accumulation of organochlorines from black-crowned night-heron eggs to chicks[J]. Environmental Toxicology and Chemistry, 14(3): 533–536.
DOI:10.1002/etc.v14:3
|
Custer T W, Dummer P M, Custer C M, et al. 2014. Contaminant exposure of birds nesting in Green Bay, Wisconsin, USA[J]. Environmental Toxicology & Chemistry, 33(8): 1832–1839.
|
Custer T W, Kaiser C M B E. 1983. Organochlorine residues in Atlantic coast black-crowned night-heron eggs, 1979[J]. Colonial Waterbirds, 6: 160–167.
DOI:10.2307/1520984
|
Custer T W, Pendleton G, Ohlendorf H M. 1990. Within-and among-clutch variation of organochlorine residues in eggs of black-crowned night-herons[J]. Environmental Monitoring & Assessment, 15(1): 83.
|
董元华, 安琼, 龚钟明, 等. 2002. 太湖湿地生态系统有机氯污染的夜鹭生物指示[J]. 应用生态学报, 2002, 13(2): 209–212.
DOI:10.3321/j.issn:1001-9332.2002.02.020 |
Dong Y H, Wang H, An Q, et al. 2004. Residues of organochlorinated pesticides in eggs of water birds from Tai Lake in China[J]. Environmental Geochemistry and Health, 26(2): 259–268.
DOI:10.1023/B:EGAH.0000039589.46698.2b
|
Fasola M, Movalli P A, Gandini C. 1998. Heavy metal, organochlorine pesticide, and PCB residues in eggs and feathers of herons breeding in northern Italy[J]. Archives of Environmental Contamination & Toxicology, 34(1): 87–93.
|
Focardi S, Fossi C, Leonzio C, et al. 1996. Persistent organochlorine residues in fish and water birds from the Biobio River, Chile[J]. Environmental Monitoring & Assessment, 43(1): 73–92.
|
Fossi C, Focardi S, Leonzio C, et al. 1984. Trace-metals and chlorinated hydrocarbons in birds' eggs from the delta of the Danube[J]. Environmental Conservation, 11(4): 345.
DOI:10.1017/S0376892900014715
|
龚钟明, 董元华, 等. 2001. 夜鹭卵中几种多氯联苯(PCBs)的残留特征[J]. 中国环境科学, 2001, 21(2): 124–127.
DOI:10.3321/j.issn:1000-6923.2001.02.008 |
Greaves A K, Letcher R J, Chen D, et al. 2016. Retrospective analysis of organophosphate flame retardants in herring gull eggs and relation to the aquatic food web in the Laurentian Great Lakes of North America[J]. Environmental Research, 150: 255–263.
DOI:10.1016/j.envres.2016.06.006
|
Henny C J, Blus L J, Krynitsky A J, et al. 1984. Current impact of DDE on black-crowned night-herons in the intermountain west[J]. Journal of Wildlife Management, 48(1): 1–13.
DOI:10.2307/3808448
|
Hothem R L, Marois K C, Wainwright S E, et al. 1995. Spatial and temporal trends of contaminants in eggs of wading birds from San Francisco Bay, California[J]. Environmental Toxicology & Chemistry, 14(8): 1319–1331.
|
Hu Y, Qi S, Yuan L, et al. 2016. Assessment of organochlorine pesticide contamination in waterbirds from an agricultural region, Central China[J]. Environ Geochem Health, 40(1): 1–13.
|
黄强, 邓合黎, 毛珂. 1995. 四川宜宾地区鸟类调查报告[J]. 动物学杂志, 1995, 30(6): 7–15.
|
Huertas D, Grimalt J O, Jover L, et al. 2016. Organochlorine compounds in purple heron eggs (Ardea purpurea) nesting in sites located around a chlor-alkali plant (Ebro River)[J]. Science of the Total Environment, 540: 211–220.
DOI:10.1016/j.scitotenv.2015.07.037
|
Klein R, Bartelsteinbach M, Koschorreck J, et al. 2012. Standardization of egg collection from aquatic birds for biomonitoring-a critical review[J]. Environmental Science & Technology, 46(10): 5273–5284.
|
Koponen J, Rantakokko P, Airaksinen R, et al. 2013. Determination of selected perfluorinated alkyl acids and persistent organic pollutants from a small volume human serum sample relevant for epidemiological studies[J]. Journal of Chromatography A, 1309(2): 48–55.
|
Lam J C W, Tanabe S, Wong B S F, et al. 2004. Trace element residues in eggs of little egret (Egretta garzetta) and black-crowned night heron (Nycticorax nycticorax) from Hong Kong, China[J]. Marine Pollution Bulletin, 48(3/4): 390–396.
|
Lam J C W, Murphy M B, Wang Y, et al. 2008. Risk assessment of organohalogenated compounds in water bird eggs from South China[J]. Environmental Science & Technology, 42(16): 6296–302.
|
李雷, 危起伟, 吴金明, 等. 2013. 长江宜宾江段渔业资源现状调查[J]. 长江流域资源与环境, 2013, 22(11): 1449–1457.
|
廖峻涛, 柳江, 赵雪冰. 2013. 长江上游向家坝库区鸟类多样性研究及生境价值评估[J]. 长江流域资源与环境, 2013, 22(6): 713–720.
|
Lucentini L, Pettine P, Stottmeister E, et al. 2013. Chemical analysis of the quality of water for human consumption:Proposal for the revision of the performance requirements in the drinking water directive 98/83/EC[J]. TrAC Trends in Analytical Chemistry, 45(2): 37–47.
|
Luo X J, Zhang X L, Liu J, et al. 2009. Persistent halogenated compounds in waterbirds from an e-waste recycling region in South China[J]. Environmental Science & Technology, 43(43): 306–311.
|
Matz A C, Parsons K C. 2004. Organochlorines in black-crowned night heron (Nycticorax nycticorax) eggs reflect persistent contamination in Northeastern US Estuaries[J]. Archives of Environmental Contamination & Toxicology, 46(2): 270–274.
|
McEwen L C, Stafford C J, Hensler G L. 1984. Organochlorine residues in eggs of black-crowned night herons from Colorado and Wyoming[J]. Environmental Toxicology and Chemistry, 3(3): 367–376.
DOI:10.1002/etc.v3:3
|
Mora M A, Durgin B, Hudson L B, et al. 2016. Temporal and latitudinal trends of p, p'-DDE in eggs and carcasses of North American birds from 1980 to 2005[J]. Environmental Toxicology & Chemistry, 35(6): 1340–1348.
|
Ohlendorf H M, Marois K C. 1990. Organochlorine and selenium in California night-heron and egret eggs[J]. Environmental Monitoring & Assessment, 15(1): 91–104.
|
Padula V, Burger J, Newman S H, et al. 2010. Metals in feathers of black-crowned night-heron (Nycticorax nycticorax) chicks from the New York Harbor Estuary[J]. Archives of Environmental Contamination & Toxicology, 59(1): 157–65.
|
Rattner B A, Mcgowan P C. 2007. Potential hazards of environmental contaminants to avifauna residing in the Chesapeake Bay Estuary[J]. Waterbirds, 30(sp1): 63–81.
DOI:10.1675/1524-4695(2007)030[0063:PHOECT]2.0.CO;2
|
Rattner B A, Melancon M J, Rice C P, et al. 1997. Cytochrome P450 and organochlorine contaminants in black-crowned night-herons from the Chesapeake Bay region, USA[J]. Environmental Toxicology & Chemistry, 16(11): 2315–2322.
|
Rattner B A, Wiemeyer S N, Blus L J. 2016. Retrospective:Adjusting contaminant concentrations in bird eggs to account for moisture and lipid loss during their incubation[J]. Bulletin of Environmental Contamination and Toxicology, 97(1): 2–3.
|
阮禄章, 张迎梅, 赵东芹, 等. 2003. 白鹭作为无锡太湖地区环境污染指示生物的研究[J]. 应用生态学报, 2003, 14(2): 263–268.
DOI:10.3321/j.issn:1001-9332.2003.02.024 |
Schmitt C J, Echols K R, Peterman P H, et al. 2018. Organochlorine chemical residues in northern cardinal (Cardinalis cardinalis) eggs from Greater Washington, DC USA[J]. Bulletin of Environmental Contamination & Toxicology, 100(6): 741–747.
|
王培潮. 1992. 鸟卵孵化生理生态学(续)[J]. 四川动物, 1992, 11(1): 21–24.
|
Wang Y, Murphy M B, Lam J C W, et al. 2011. Polychlorinated biphenyls and organochlorine pesticides in local waterbird eggs from Hong Kong:Risk assessment to local waterbirds[J]. Chemosphere, 83(7): 891–896.
DOI:10.1016/j.chemosphere.2011.02.073
|
邢新丽, 祁士华, 张凯, 等. 2009. 地形和季节变化对有机氯农药分布特征的影响-以四川省成都经济区为例[J]. 长江流域资源与环境, 2009, 18(10): 985–991.
DOI:10.3969/j.issn.1004-8227.2009.10.018 |
Ye M S, Zhang R B, Chen L H, et al. 2016. Detection of persistent organochlorine pollutants in eggs of Antarctic seabirds based on gas chromatography and their ecological environment significance[J]. Meteorological & Environmental Research, 7(1): 48–51.
|
张国钢, 梁伟, 楚国忠. 2007. 海南3种鹭越冬行为的比较[J]. 动物学杂志, 2007, 42(6): 125–130.
DOI:10.3969/j.issn.0250-3263.2007.06.021 |
Zhou Y, Asplund L, Yin G, et al. 2016a. Extensive organohalogen contamination in wildlife from a site in the Yangtze River Delta[J]. Science of the Total Environment, 554-555: 320–328.
DOI:10.1016/j.scitotenv.2016.02.176
|
Zhou Y, Yin G, Asplund L, et al. 2016b. A novel pollution pattern:Highly chlorinated biphenyls retained in black-crowned night heron (Nycticorax nycticorax) and whiskered tern (Chlidonias hybrida) from the Yangtze River Delta[J]. Chemosphere, 150: 491–498.
DOI:10.1016/j.chemosphere.2015.11.112
|
 2019, Vol. 39
2019, Vol. 39


