2. 中国科学院生态环境研究中心, 饮用水科学与技术重点实验室, 北京 100085
2. Key Laboratory of Drinking Water Science and Technology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085
近年来, 药物残留在各种环境基质如地表水、地下水和土壤中被广泛检出(Langford et al., 2015; Vergeynst et al., 2015; Wang et al., 2015; Bradley et al., 2017), 对生态环境带来了严峻的挑战.药物在生物体内葡萄糖苷酸转移酶、磺酸基转移酶等的作用下, 会加成葡萄糖醛酸、酸盐和乙酰基等亲水基团(Nakanishi et al., 2012; Alherz et al., 2017; Brown et al., 2018), 从而形成代谢产物.据报道, 被人和动物使用的药物有85%以上会以原药或代谢产物的形式排出体外, 从而进入环境(Tong et al., 2011).相较于母体药物而言, 国内外目前对于环境中药物代谢产物的研究相对较少, 因此, 可能导致对药物风险的低估或出现难以解释的调查结果.例如, 诸多研究都报道了卡马西平(CBZ)在污水处理厂中的“负去除率”(Miao et al., 2005; Nakada et al., 2007; 周海东等, 2007; Vergeynst et al., 2015), 可能原因在于忽略了其代谢产物在污水处理过程中转化为母体化合物, 从而导致CBZ在出水中的浓度高于进水.城市污水处理厂(Wastewater Treatment Plants, WWTPs)作为环境中药物污染的重要来源之一, 近年来涌现了大量有关WWTPs中药物浓度的调查数据(Pedersen et al., 2003; Clara et al., 2005), 但由于缺乏简便可靠的检测方法, 关于其中药物代谢产物的分布及去除等方面的研究十分有限, 有关母体药物与代谢产物同步分析检测的研究更是少见.
针对以上问题, 本文选取了磺胺甲恶唑(SMX)、卡马西平(CBZ)及其5种代谢产物(醋酸磺胺甲恶唑(N-AcSMX)、磺胺甲恶唑-D-葡糖苷酸(SMX-DG)、卡马西平环氧化物(CBZ-EPO)、2-羟基卡马西平(2OH-CBZ)、10, 11-二氢-10-羟基卡马西平(LCBZ))为研究对象, 针对城市污水处理厂污水及污泥分别开发了母体及代谢产物的同步检测方法.在此基础上, 对两家城市污水处理厂污水及污泥进行采样分析, 揭示其中母体与代谢产物的分布特征.以期为进一步提升对城市污水处理厂中药物的赋存、去除及风险的认识提供方法基础.
2 实验部分(Experimental methods) 2.1 仪器和试剂仪器:超高效液相色谱-质谱联用仪(Agilent 1290/6420, 美国Agilent);离心机(TGL-16G, 湖南星科);冷冻干燥机(FD-1, 北京博医康);24管防交叉污染SPE装置(美国Supelco);氮吹仪(SE812, 北京莱博).
试剂:SMX和CBZ购自美国Sigma-Aldrich公司; 2OH-CBZ购自加拿大TRC公司;CBZ-EPO、LCBZ、N-AcSMX和SMX-DG购自百灵威公司;玻璃纤维滤膜(GF/B 1 μm)购自英国Whatman公司;Na2EDTA·2H2O、一水合柠檬酸、硫酸和盐酸均为分析纯, 购自国药集团化学试剂有限公司;甲醇(MeOH)、乙腈(ACN)、丙酮(ACE)和二氯甲烷(DCM)均为HPLC级, 购自比利时Fisher公司;超纯水(MQ)产自Milli-Q净水系统(Advantage 10, Millipore), 电阻率为18 mΩ.
2.2 标准溶液精确称取各标准品0.01 g, 用MeOH溶解并定容至100 mL, 配置成100 mg·L-1的储备液并置于-20 ℃保存.一水合柠檬酸、Na2EDTA·2H2O和盐酸溶液浓度分别为0.2、0.5和0.5 mol·L-1, 硫酸配置成40%溶液备用.McIlvaine缓冲溶液:称取一水合柠檬酸21.0 g、Na2HPO4 17.75 g和Na2EDTA·2H2O 60.5 g, 溶解于1.625 L水中, 调节pH值至4.00±0.05.
2.3 样品前处理污水用玻璃纤维膜过滤后, 取500 mL加入40%的硫酸, 将pH调节至2.5~3.0, 然后加入500 μL 0.5 mol·L-1的Na2EDTA溶液螯合水中的金属离子.依次用5 mL MeOH、5 mL 0.5 mol·L-1盐酸和5 mL超纯水对SPE柱(Waters Oasis HLB, 500 mg/6 mL)进行活化.将处理后的水样以4~5 mL·min-1的流速通过SPE柱, 之后依次用5 mL 5%甲醇水和5 mL超纯水对SPE柱进行清洗.真空抽吸小柱至干燥, 用10 mL洗脱液进行淋洗, 洗脱液用锥形管收集, 置于35 ℃水浴中氮吹至干.先后向锥形管中加入400 μL MeOH和600 μL超纯水复溶样品, 经0.22 μm针式过滤器过滤后进入UPLC-MS/MS分析.
污泥在6000 r·min-1条件下离心, 取固相冷冻干燥后磨成粉末并过筛.精确称取0.5 g置于30 mL玻璃离心管中, 加入10 mL萃取液, 旋涡振荡1 min, 使其混合均匀;然后将样品超声10 min, 中间不断摇晃离心管防止样品沉淀;随后将样品在5500 r·min-1条件下离心8 min, 将上清液倒出收集.重复萃取3次, 步骤同上.合并萃取液加水稀释至300 mL, 加入500 μL 0.5 mol·L-1 Na2EDTA溶液, 随后进行SPE, 步骤与污水样品相同.
2.4 色谱-质谱条件色谱柱:Agilent Zorbax SB-C18 (100 mm× 2.1 mm, 1.8 μm, Agilent);柱温:30 ℃;进样量:3 μL;流速:0.2 mL·min-1;流动相:0.2%甲酸(A)和乙腈(B).采用梯度洗脱程序:0 min, 30%B;1 min, 30%B;3 min, 50%B;6 min, 50%B;8 min, 80%B;10 min, 80%B;10.1 min, 30%B;11 min, 30%B.离子源:ESI+;扫描模式:多重反应监测模式(MRM);毛细电压:4.0 kV;干燥气温度:350 ℃;干燥气流速:11 L·min-1;雾化气压力:241 kPa.其余参数见表 1.
| 表 1 MRM模式下7种目标物质的质谱参数 Table 1 MS/MS parameters for the target compounds under MRM mode |
本实验采用超声溶剂萃取(Ultrasonic solvent extraction, USE)、固相萃取法(Solid phase extraction, SPE)作为样品前处理步骤, 为提高目标物的回收率同时尽可能减少背景基质的干扰, 需对USE萃取液和SPE洗脱液进行优化.
3.1.1 USE萃取液优化据报道, 弱酸性溶液和有机溶剂的混合液用作萃取液通常对固体样品中的药物类残留有较好的提取效果(Chen et al., 2011).本实验对比了萃取液A:0.2 mol·L-1的柠檬酸溶液:ACN =1:1(体积比)(pH=4.0)、萃取液B:0.1%甲酸:MeOH=1:1(体积比)(pH=2.7)、萃取液C:McIlvaine缓冲液:MeOH =1:1(体积比)(pH=4.4)和萃取液D:0.2 mol·L-1的柠檬酸溶液:MeOH=1:1(体积比)(pH=4.0)对7种目标物的萃取效果, 回收率结果如图 1a所示.由图可知, 萃取液D对所有目标物均有较好的提取效果, 回收率为76%~146%, 萃取液A和C对N-AcSMX和SMX-DG的萃取效率较低, 回收率小于50%, 萃取液B则无法从样品中提取出N-AcSMX和SMX-DG.
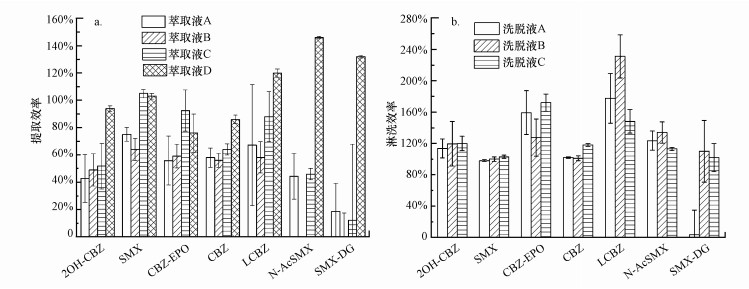 |
| 图 1 不同萃取液对目标物的提取效率(a)和不同淋洗液对目标物的淋洗效率(b) Fig. 1 Extraction efficiency for target compounds by different extraction solvents(a) and rinse efficiency for target compounds by different eluents(b) |
由于7种目标物的极性差异较大, 因此, 选择3种不同极性的洗脱液考察其对目标物的回收效果.如图 1b所示, 洗脱液A(ACN)未能将SMX-DG洗脱, 其回收率小于10%;使用洗脱液B(V(DCM):V(ACE):V(MeOH)=3:2:5)时, LCBZ的回收率偏高, 且RSD大于20%;洗脱液C(MeOH)对目标物均有较好的淋洗效果, 回收率介于102%~172%之间.
3.2 色谱分离及质谱测定条件优化为改善目标物的峰形, 提高分析灵敏度, 通常会向流动相中添加甲酸、氨水等离子化助剂(唐才明等, 2009).本实验发现加入0.2%甲酸会令目标物具有更好的离子化效果, 并能较好地改善目标物的峰形.因此, 本方法采用0.2%甲酸水作为流动相A, 乙腈作为流动相B, 使用梯度淋洗的方式对目标物质进行分离.如图 2所示, 当采用“2.4节”所示的梯度淋洗程序时, 7种目标物能获得较好的分离效果.此外, 实验发现当柱温为30 ℃时, 目标物质的分离度和峰形最为理想.
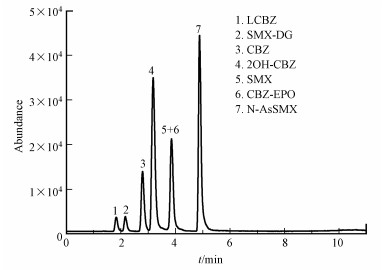 |
| 图 2 7种目标物(100 μg·L-1)的总离子色谱图 Fig. 2 Total-ion MRM chromatograms of seven target compounds(100 μg·L-1) in ultrapure water |
配置一系列浓度为1、5、10、30、50、100、300、500 μg·L-1的混合标准溶液以建立标准曲线, 结果显示, 各目标物标准曲线的R2均大于0.99.加标回收率可反映整个方法流程的准确度, 如表 2所示, 目标物在纯水、污水及污泥中的回收率范围分别为107%~137%、91%~217%和86%~172%, 其RSD在1%~18%之间.此外, 针对CBZ-EPO等表现出较高回收率的目标物, 进行了日间的加标回收实验, 结果显示RSD均在14%以下.参照美国EPA规定, 对环境样品来说, 当平行样品检测结果的RSD值小于20%时, 该方法可被认为具有较高的精密度和重现性.
| 表 2 目标物质的方法回收率、精度及检出限 Table 2 Recoveries, precision and MQLs for the target compounds |
通过稀释标准溶液获得UPLC-MS/MS信号信噪比为10时的目标物浓度, 确定其为仪器定量限(Limit of Quantitation, LOQ).方法定量限(Method Quantitation Limit, MQL)与仪器定量限之间的关系由浓缩倍数和回收率确定, 具体计算方法如下:
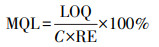
|
(1) |
式中, RE代表目标物质在相应基质中的回收率, C为样品的浓缩倍数.如表 2所示, 纯水、污水和污泥中各物质的MQL分别为0.20~3.05 ng·L-1、0.17~2.42 ng·L-1和0.20~2.85 μg·kg-1.
3.4 实际样品测定采集天津市两座城市污水处理厂的进水、二沉出水和总出水样品, 采用本方法对其中SMX、CBZ和代谢物的浓度进行测定.污水厂进水主要为生活污水, 厂A日处理量为20×104 m3·d-1, 采用A/O工艺, 深度处理工艺为反硝化深床滤池;厂B日处理量为55×104 m3·d-1, 采用A/A/O-A/O工艺, 三级工艺为UV消毒.两座污水厂污水及污泥中的目标物检出浓度见表 3.结果显示, 污水及污泥中分别检出6种及4种目标物, 其浓度分别为3.92~667 ng·L-1及0.41~2.74 μg·kg-1, 可见本文所关注的药物及代谢物主要存在于污水中.
| 表 3 城市污水处理厂污水和污泥中7种目标物质的检出浓度(n=3) Table 3 Concentrations of target compounds in wastewater and sludge samples from two WWTPs(n=3) |
SMX在两厂进水中的浓度分别为107和129 ng·L-1, 该结果与国内污水处理厂的检出浓度相当(周佳虹等, 2017; Ben et al., 2018);污水处理工艺未能有效削减SMX浓度, 其在出水中浓度仍较高, 在厂B出水中的浓度甚至高于进水.SMX的两种代谢物中, SMX-DG仅在A厂进水中检出, 浓度为3.92 ng·L-1, N-AcSMX则未被检出, 说明SMX在污水中主要以母体形式存在.CBZ在两厂进水中的浓度略低于SMX, 分别为25.5和75.3 ng·L-1, 但其浓度随污水处理流程逐渐升高, 在出水中的浓度均高于进水.据文献报道, 无论使用哪种生物处理工艺, CBZ都很难被完全去除(Jelic et al., 2011; Yang et al., 2017), 出水浓度高于进水浓度而造成的“负去除率”现象也很常见(Miao et al., 2005; Tran et al., 2017).由于CBZ分子结构中缺少供电子基团(如甲氧基、酚羟基或乙酰基等), 导致其在生物处理工艺中呈现出较低的去除率(Tran et al., 2017; Park et al., 2018);而CBZ的代谢物在污水处理过程中转化为母体(Wang et al., 2014)也可能是造成其去除率波动的重要原因(Miao et al., 2005; Radjenović et al., 2009).本研究所选取的3种CBZ代谢物在两座污水厂污水中均被检出, 其中, CBZ-EPO和LCBZ的浓度分别为12.6~36.9 ng·L-1和50.0~667 ng·L-1, 其浓度与母体CBZ相当甚至更高.Miao和Zhang等曾在城市污水处理厂污水中检出了CBZ的代谢产物反式10, 11-二氢-10, 11-二羟基卡马西平, 且检出浓度高于CBZ(Miao et al., 2003; Zhang et al., 2008).据报道, 药物代谢产物的毒性可能比母体要强(郭倩等, 2019), 因此, 有必要针对城市污水处理厂污水中的药物代谢产物的分布及去除开展进一步研究.
4 结论(Conclusions)1) 实际污水厂样品检测结果表明, 药物代谢产物在污水处理厂中广泛存在, 部分物质浓度与母体相当甚至高于母体.
2) 污水处理流程未能有效削减药物及其代谢产物浓度, 出现“负去除率”的现象.可见, 在针对城市污水厂药物污染物的污水处理工艺开发及排放风险评估的研究中, 应同时对药物代谢产物给予密切关注.
Alherz F A, Almarghalani D A, Hussein N A, et al. 2017. A reappraisal of the 6-O-desmethylnaproxen-sulfating activity of the human cytosolic sulfotransferases[J]. Canadian Journal of Physiology and Pharmacology, 95(6): 647-651. DOI:10.1139/cjpp-2016-0403 |
Ben W W, Zhu B, Yuan X J, et al. 2018. Occurrence, removal and risk of organic micropollutants in wastewater treatment plants across China:Comparison of wastewater treatment processes[J]. Water Research, 130: 38-46. DOI:10.1016/j.watres.2017.11.057 |
Bradley P M, Journey C A, Romanok K M, et al. 2017. Expanded target-chemical analysis reveals extensive mixed-organic-contaminant exposure in U.S.Streams[J]. Environmental Science & Technology, 51(9): 4792-4802. |
Brown A K, Wong C S. 2018. Distribution and fate of pharmaceuticals and their metabolite conjugates in a municipal wastewater treatment plant[J]. Water Research, 144: 774-783. DOI:10.1016/j.watres.2018.08.034 |
Chen F, Ying G, Kong L, et al. 2011. Distribution and accumulation of endocrine-disrupting chemicals and pharmaceuticals in wastewater irrigated soils in Hebei, China[J]. Environmental Pollution, 159(6): 1490-1498. DOI:10.1016/j.envpol.2011.03.016 |
Clara M, Strenn B, Gans O, et al. 2005. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants[J]. Water Research, 39(19): 4797-4807. DOI:10.1016/j.watres.2005.09.015 |
郭倩, 唐光贝, 彭稳, 等. 2019. 卡马西平及其衍生物的环境行为及其去除研究进展[J]. 环境化学, 38(8): 1708-1715. |
Jelic A, Gros M, Ginebreda A, et al. 2011. Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment[J]. Water Research, 45(3): 1165-1176. DOI:10.1016/j.watres.2010.11.010 |
Langford K H, Reid M J, Fjeld E, et al. 2015. Environmental occurrence and risk of organic UV filters and stabilizers in multiple matrices in Norway[J]. Environment International, 80: 1-7. DOI:10.1016/j.envint.2015.03.012 |
Miao X S, Metcalfe C D. 2003. Determination of carbamazepine and its metabolites in aqueous samples using liquid chromatography-electrospray tandem mass spectrometry[J]. Analytical Chemistry, 75(15): 3731-3738. DOI:10.1021/ac030082k |
Miao X S, Yang J J, Metcalfe C D. 2005. Carbamazepine and its metabolites in wastewater and in biosolids in a municipal wastewater treatment plant[J]. Environmental Science & Technology, 39(19): 7469-7475. |
Nakada N, Shinohara H, Murata A, et al. 2007. Removal of selected pharmaceuticals and personal care products (PPCPs) and endocrine-disrupting chemicals (EDCs) during sand filtration and ozonation at a municipal sewage treatment plant[J]. Water Research, 41(19): 4373-4382. DOI:10.1016/j.watres.2007.06.038 |
Nakanishi K, Katagi M, Zaitsu K, et al. 2012. Simultaneous enantiomeric determination of MDMA and its phase Ⅰ and phase Ⅱ metabolites in urine by liquid chromatography-tandem mass spectrometry with chiral derivatization[J]. Analytical and Bioanalytical Chemistry, 404(8): 2427-2435. DOI:10.1007/s00216-012-6385-9 |
Park S, Lee W. 2018. Removal of selected pharmaceuticals and personal care products in reclaimed water during simulated managed aquifer recharge[J]. Science of the Total Environment, 640-641: 671-677. DOI:10.1016/j.scitotenv.2018.05.221 |
Pedersen J A, Yeager M A, Suffet I H. 2003. Xenobiotic organic compounds in runoff from fields irrigated with treated wastewater[J]. Journal of Agricultural and Food Chemistry, 51(5): 1360-1372. DOI:10.1021/jf025953q |
Radjenović J, Petrović M, Barceló D. 2009. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment[J]. Water Research, 43(3): 831-841. DOI:10.1016/j.watres.2008.11.043 |
Snyder S A, Westerhoff P, Yoon Y, et al. 2003. Pharmaceuticals, personal care products, and endocrine disruptors in water:Implications for the water industry[J]. Environmental Engineering Science, 20(5): 449-469. DOI:10.1089/109287503768335931 |
唐才明, 黄秋鑫, 余以义, 等. 2009. 高效液相色谱-串联质谱法对水环境中微量磺胺、大环内酯类抗生素、甲氧苄胺嘧啶与氯霉素的检测[J]. 分析测试学报, 28(8): 909-913. DOI:10.3969/j.issn.1004-4957.2009.08.007 |
Tong C L, Zhuo X J, Guo Y. 2011. Occurrence and risk assessment of four typical fluoroquinolone antibiotics in raw and treated sewage and in receiving waters in Hangzhou, China[J]. Journal of Agricultural and Food Chemistry, 59(13): 7303-7309. DOI:10.1021/jf2013937 |
Tran N H, Gin K Y-H. 2017. Occurrence and removal of pharmaceuticals, hormones, personal care products, and endocrine disrupters in a full-scale water reclamation plant[J]. Science of the Total Environment, 599-600: 1503-1516. DOI:10.1016/j.scitotenv.2017.05.097 |
Vergeynst L, Haeck A, De Wispelaere P, et al. 2015. Multi-residue analysis of pharmaceuticals in wastewater by liquid chromatography-magnetic sector mass spectrometry:Method quality assessment and application in a Belgian case study[J]. Chemosphere, 119: S2-S8. DOI:10.1016/j.chemosphere.2014.03.069 |
Wang J, Gardinali P R. 2014. Identification of phase Ⅱ pharmaceutical metabolites in reclaimed water using high resolution benchtop Orbitrap mass spectrometry[J]. Chemosphere, 107: 65-73. DOI:10.1016/j.chemosphere.2014.03.021 |
Wang Z, Zhang X H, Huang Y, et al. 2015. Comprehensive evaluation of pharmaceuticals and personal care products (PPCPs) in typical highly urbanized regions across China[J]. Environmental Pollution, 204: 223-232. DOI:10.1016/j.envpol.2015.04.021 |
Yang Y, Ok Y S, Kim K H, et al. 2017. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants:A review[J]. Science of the Total Environment, 596: 303-320. |
Zhang Y, Geissen S U, Gal C. 2008. Carbamazepine and diclofenac:removal in wastewater treatment plants and occurrence in water bodies[J]. Chemosphere, 73(8): 1151-1161. DOI:10.1016/j.chemosphere.2008.07.086 |
周海东, 黄霞, 文湘华. 2007. 城市污水中有关新型微污染物PPCPs归趋研究的进展[J]. 环境工程学报, 1(12): 1-9. DOI:10.3969/j.issn.1673-9108.2007.12.001 |
周佳虹, 张翔宇, 李茹莹. 2017. 抗生素在污水处理系统中的迁移分布研究进展[J]. 环境科学与技术, 40(8): 77-86. |
 2020, Vol. 40
2020, Vol. 40


