2. 广东省大气环境与污染控制重点实验室, 广州 510006;
3. 清华大学盐城环境工程技术研发中心, 盐城 224000
2. Guangdong Provincial Key Laboratory of Atmospheric Environment and Pollution Control, Guangzhou 510006;
3. Tsinghua-Yancheng Environmental Engineering Technology Research and Development Center, Yancheng 224000
燃煤过程如发电、工业锅炉、炼焦等在消耗大量煤炭资源的同时会排放种类复杂且总量巨大的污染物, 包括粉尘、颗粒物(PM)、氮氧化物(NOx)和二氧化硫(SO2)等常规污染物, 与此同时, 该过程还会产生挥发性有机污染物(VOCs), 其危害性同样不容忽视(Hao et al., 2017; Xu et al., 2018; Shao et al., 2019).相关研究表明, 燃煤电厂排放的VOCs主要包含烷烃、芳香烃及多环芳烃(PAHs), 其中, 单环芳烃(苯及其单环衍生物)为主要成分, 占总VOCs的50%~90%(Moreira Dos Santos et al., 2004; Cheng et al., 2019; Liu et al., 2019).VOCs作为臭氧和PM2.5的重要前驱体, 对生态环境和人体健康构成重大威胁(Ye et al., 2018; Qin et al., 2020).目前国内外有关VOCs的去除主要采用催化氧化技术, 并且主要针对工业源VOCs排放和室内VOCs的治理.然而, 与工业源VOCs排放特征相比, 燃煤烟气中的挥发性有机物具有环境成分复杂(含有NH3、NO和SO2等)、浓度低(1~20 mg · m-3)、烟气量大(百万级)、毒性高等特点, 无法直接应用现有工业源VOCs吸附和催化氧化技术及材料(Shi et al., 2015; Yan et al., 2016).单独安装NO和VOCs去除装备占地面积大且成本高, 出于技术可行性和经济成本考虑, 在选择性催化还原(SCR)装置内实现NO和VOCs的同步去除将是有效的解决办法.
根据实际工况下在线监测数据显示, 烟气中NO浓度远高于VOCs浓度, 因此, 基于商用钒钛基脱硝催化剂设计甲苯和NO同步去除催化剂将更具应用前景.研究发现, 过渡金属改性钒钛基催化剂在VOCs和NO同步去除上具有优异性能.Fe改性材料比表面积大、氧化还原性强, 具有较强的抗烟气中毒能力(王玉亭等, 2020).Ce改性材料由于表面具有合适的酸性位点、良好的氧化还原性能、丰富的表面氧物种等特性, 因而表现出更优异的氧化甲苯和还原NO的能力(Chen et al., 2020; Chen et al., 2020).Mo改性钒钛催化剂可以表现出优异的氯苯与NOx同步去除能力(Zhang et al., 2015; Huang et al., 2021).
基于实际工业应用考虑及本课题组前期研究成果, 本研究采用简易可行的单组分浸渍法和多组分浸渍法制备一系列Cu/Fe/Mo改性的钒钛基整体式催化剂, 选用甲苯和NO作为探针分子, 在高浓度烟气氛围([NH3]=[NO]=0.5‰, [SO2]=1‰)中考察不同整体式催化剂制备工艺及浸渍液浓度对其在模拟燃煤烟气中对甲苯和NO同步去除的性能.利用MultiGas检测器同时在线监测甲苯、CO、CO2、NO、N2O、NO2等污染物、目标产物和副产物浓度变化, 并利用相关表征分析改性催化剂的理化性质, 以期得到最优的适应燃煤烟气的改性催化剂制备工艺及配方.
2 实验部分(Experimental) 2.1 材料实验使用的钒钛蜂窝材料购于江苏省中创清源有限公司, 偏钒酸铵(NH4VO3)、硝酸铜(Cu(NO3)2)、钨酸铵((NH4)10H2(W2O7)6)、钼酸铵((NH4)2MoO4)、草酸(C2H2O4 · 2H2O)及硝酸铁(FeN3O9 · 9H2O)等化学试剂均为分析纯, 购于上海麦克林生化科技有限公司.
2.2 催化剂的制备 2.2.1 单组分浸渍法整体式催化剂的制备以Cu改性样品为例, 在去离子水中加入硝酸铜制备成前驱体溶液, Cu占二氧化钛的比例分别为0.5%和5%(质量分数), 按照比例加入去离子水配置成相应质量分数的浸渍液.将钒钛蜂窝材料去除吸附的各种杂质, 用空气吹扫, 浸泡于浸渍溶液中超声搅拌浸渍, 然后吹扫残余悬浮液, 在基体表面形成均匀的薄膜, 干燥;重复上述浸渍过程3次;将样品过夜陈化, 干燥;最后将样品块在马弗炉中煅烧, 得到整体式催化剂, 其中, 煅烧温度为450 ℃, 煅烧时间为12 h, 标记为Cu-x(x为浸渍液浓度).Fe/Mo改性样品制备方法同上, 其中, Fe的负载量分别为0.5%和5%, Mo的负载量分别为0.1%、0.5%和3%.
2.2.2 多组分浸渍法整体式催化剂的制备以Cu改性样品为例, 在去离子水中加入硝酸铜、偏钒酸铵、钨酸铵和草酸, 添加顺序为加入草酸溶解后, 加入偏钒酸铵和钨酸铵, 接着再加入硝酸铜, 其中, 钒、钨、铜占二氧化钛的比例分别为3.5%、2.5%、5%(质量分数).将钒钛蜂窝材料去除吸附的杂质后泡于多组分浸渍液中超声搅拌, 其余步骤与单组分浸渍法相同, 标记为Cu-VW.Fe/Mo改性样品制备方法同上, 其中, Fe占载体的5%, Mo占载体的3%(质量分数).改性催化剂煅烧前后对比如图 1所示, 与空白样品(钒钛蜂窝样品)相比, 改性组分均成功负载.
 |
| 图 1 改性催化剂样品煅烧前后对比图 Fig. 1 Comparison of modified samples before and after calcination |
通过X射线衍射技术来测定各催化材料的物相结构, 所用仪器为D8 ADVANCE型X射线衍射仪(Bruker-AXS公司, 德国), 以Cu Kα为射线源(λ=0.15418 nm), 电压40 kV, 电流40 mA, 扫描范围为5°~80°, 扫描步长为0.02°.使用ASAP 2020 M全自动表面分析仪(Micromeritics, 美国)测试样品的比表面积: 取100 mg样品装入样品管中, 在300 ℃下真空脱气3 h;采用静态吸附法, 在液氮恒温条件下(77 K)进行测试, 得到吸附脱附等温线及BJH吸脱附孔体积分布曲线, 样品的比表面积使用Brunauer-Emmett-Teller方程计算获得.利用扫描电子显微镜(SEM)观察样品微观形貌, 所用仪器为德国ZEISS公司生产的Ultra55型设备.
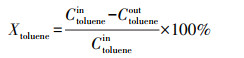
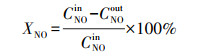
2.4 催化剂活性评价改性整体式催化剂经切块、磨碎、造粒, 制成40~60目粉末催化剂, 在350 ℃下测试评价催化剂对甲苯与NO的同步去除效率, 评价系统如图 2所示.测试条件如下: 甲苯浓度为0.05‰, 催化剂用量为100 mg, 反应温度为350 ℃, 空速为120000 h-1, 反应气氛为模拟的燃煤烟气, 其中, SO2浓度为1‰, NO浓度为0.5‰, NH3浓度为0.5‰, 5% O2, N2为平衡气体;采用MKS公司的MultiGas2030红外傅立叶变换(FTIR)光谱分析仪在线同时检测甲苯、CO、CO2、NO、N2O、NO2、NH3、SO2的实时浓度, 各气体组分每5 s取一个数值.甲苯转化率(Xtoluene)、COx([COx]=[CO]+[CO2])选择性(SCOx)、NO转化率(XNO)和N2选择性(SN2)计算公式如下(Liu et al., 2019; Zhang et al., 2020):
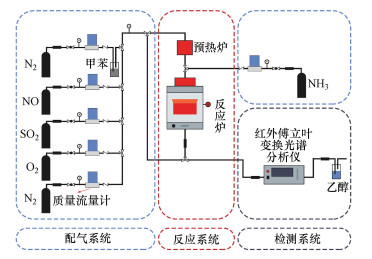 |
| 图 2 甲苯和NO同步去除性能评价装置图 Fig. 2 Diagram of evaluation device for simultaneous removal of toluene and NO |
|
(1) |
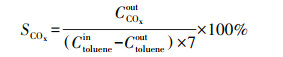
|
(2) |
|
(3) |
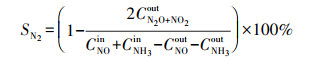
|
(4) |
改性整体式催化剂XRD谱图如图 3所示, 用以研究材料的晶相和结晶度.由图可知, 所有样品均呈现明显的锐钛矿TiO2衍射峰(Zeng et al., 2019; 梁珑等, 2020), 但改性样品均观察不到改性组分(Mo/Cu/Fe)的衍射峰, 这可能是因为其含量太低, 且均已良好分散到TiO2载体的孔道中.Fe改性样品载体衍射峰变弱, 可能是由于活性组分与载体间存在相互作用诱导晶相结构发现变化, 使得对应晶相的晶粒尺寸更小, 分散度越高, 进而表现出最优的活性和选择性(Wang et al., 2021).
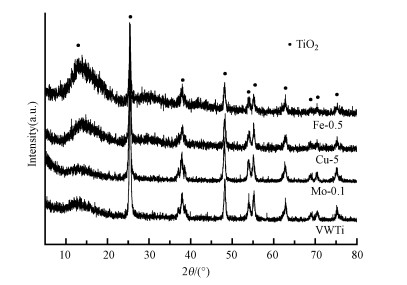 |
| 图 3 改性整体式催化剂XRD谱图 Fig. 3 XRD patterns of modified monolithic catalysts |
对不同改性样品进行SEM测试, 从图 4中可以看出, 在负载少量过渡金属氧化物后, 催化剂在纳米尺度下呈现出清晰的微球状结构.从EDS结果可以看出, 所有样品均含有V、W、O、Ti元素, 且均具有改性组分所对应的元素.Mapping结果表明所有元素均匀分散在改性样品的表面.
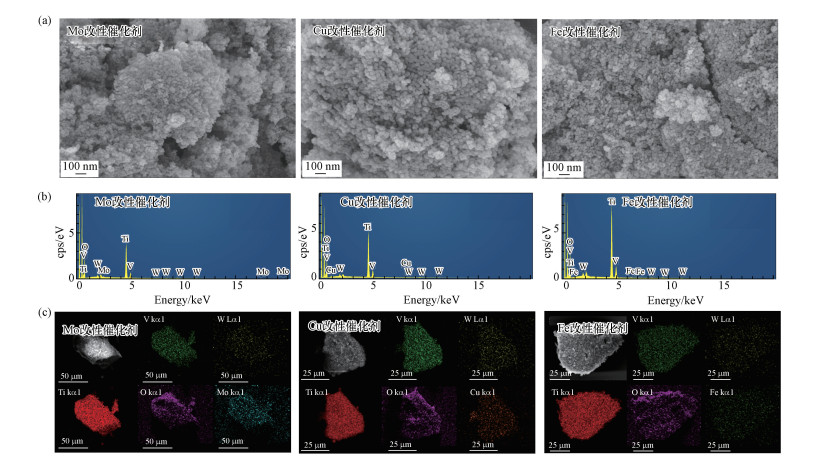 |
| 图 4 改性整体式催化剂SEM-EDS-Mapping图 (a.SEM, b.EDS, c.Mapping) Fig. 4 SEM-EDS-Mapping images of modified monolithic catalysts (a.SEM, b.EDS, c.Mapping) |
催化反应第一步需要将反应物吸附在催化剂表面, 因此, 研究催化剂的孔结构具有十分重要的意义(Yang et al., 2019; Shao et al., 2020).本研究通过N2吸附脱附实验测定了改性后催化材料的比表面积、孔容和平均孔径, 具体数据列于表 1.如图 5所示, 所有材料均表现为具有H3回滞环的Ⅳ型吸附等温线, 表明材料具有介孔结构(He et al., 2019; Wang et al., 2021).多组分浸渍法制备的催化剂比表面积和孔容均小于单组分浸渍法制备的材料, 说明多组分浸渍法容易堵塞催化剂孔道.在不同活性组分改性催化剂中, Fe改性钒钛基催化剂具有最高的比表面积和最大的孔容, 这有利于活性位点的均匀分散, 进而提高反应活性.
| 表 1 改性催化剂的孔结构 Table 1 Pore structure of modified catalysts |
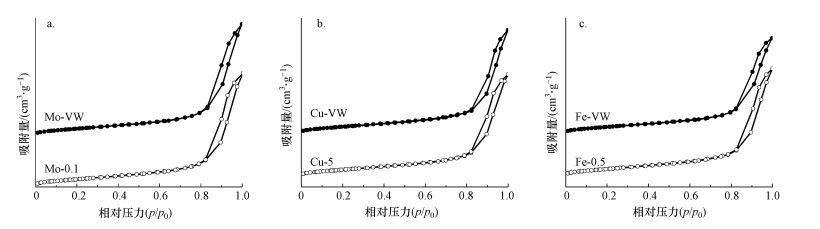 |
| 图 5 改性整体式催化剂N2吸附脱附曲线 (a.Mo, b.Cu, c.Fe) Fig. 5 N2 adsorption and desorption curves of modified monolithic catalysts (a.Mo, b.Cu, c.Fe) |
采用单组分浸渍法和多组分浸渍法制备Mo、Cu、Fe改性钒钛整体式催化剂, 探究整体式催化剂制备方法对其在模拟燃煤烟气中对甲苯与NO同步去除性能的影响, 以获得最优的整体式催化剂制备方法.由图 6可知, 单组分浸渍法比多组分浸渍法表现出更优异的活性和选择性.其中, 单组分浸渍法制备的Mo改性整体式催化剂对甲苯去除率为93.5%, NO去除率为93.1%, COx和N2选择性分别为52.2%和91.6%.单组分浸渍法制备的Cu改性整体式催化剂对甲苯去除率为99.0%, NO去除率为94.0%, COx和N2选择性分别为86.7%和82.9%.单组分浸渍法制备的Fe改性整体式催化剂对甲苯去除率为98.3%, NO去除率为93.9%, COx和N2选择性分别为77.5%和96.5%.结合改性催化剂对甲苯与NO同步去除的活性和选择性两方面考虑, 选取单组分浸渍法来制备Mo、Cu、Fe改性钒钛基整体式催化剂.
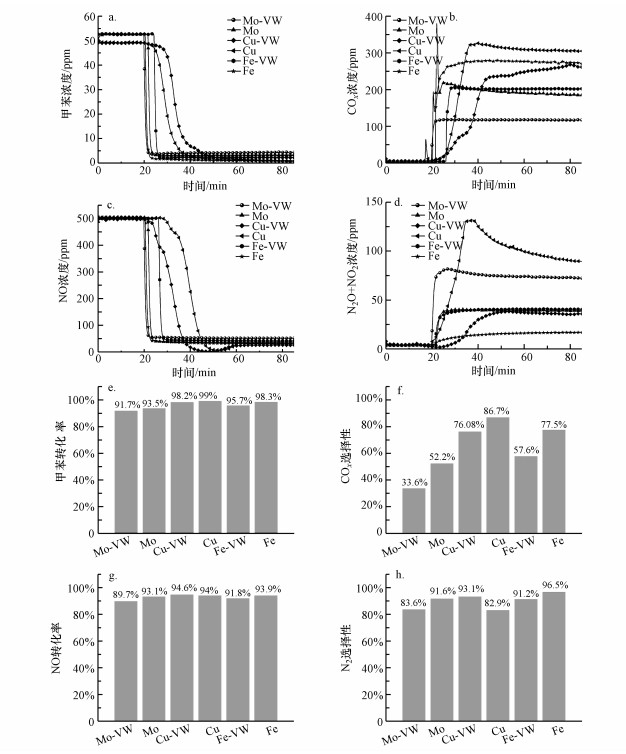 |
| 图 6 浸渍工艺对改性整体式催化剂在模拟烟气中对甲苯和NO同步去除的影响 (a.甲苯浓度, b.COx浓度, c.NO浓度, d.N2O+NO2浓度, e.甲苯转化率, f.COx选择性, g.NO转化率, h.N2选择性) Fig. 6 Effect of impregnation process on simultaneous removal of toluene and NO in simulated flue gas over modified monolithic catalysts (a. concentration of toluene, b. concentration of COx, c. concentration of NO, d. concentration of N2O+NO2, e. conversion of toluene, f. selectivity of COx, g. conversion of NO, h. selectivity of N2) |
为了研究Mo浸渍液浓度对催化剂活性、N2和COx选择性的影响, 采用单组分浸渍法制备了Mo浸渍液浓度分别为0.1%、0.5%、3%的3种Mo改性整体式催化剂, 其催化剂活性和选择性见图 7.由图可知, 反应稳定60 min后, 浸渍液浓度为0.1%的Mo改性整体式催化剂上甲苯、NO和N2O+NO2浓度最低, COx浓度最高.这表明Mo-0.1催化剂对甲苯和NO去除率最高, 对COx和N2选择性最优.当浸渍液浓度提高后活性不升反降, 其原因可能是当催化剂负载量增加后, 导致整体式催化剂的部分孔隙堵塞, 催化反应接触面积减小.
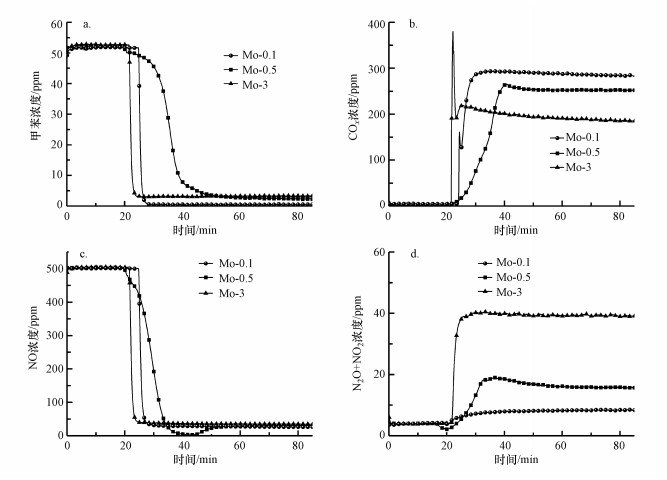 |
| 图 7 Mo浸渍液浓度对改性整体式催化剂在模拟烟气中对甲苯和NO同步去除的影响 (a.甲苯浓度, b.COx浓度, c.NO浓度, d.N2O+NO2浓度) Fig. 7 Effect of Mo impregnation solution concentration on simultaneous removal of toluene and NO in simulated flue gas over modified monolithic catalysts (a. concentration of toluene, b. concentration of COx, c. concentration of NO, d. concentration of N2O+NO2) |
为研究Fe浸渍液浓度对催化剂活性和选择性的影响, 采用单组分浸渍法制备了Fe浸渍液浓度分别为0.5%、5%的两种Fe改性整体式催化剂.由图 8可知, 反应稳定60 min后, Fe浸渍液浓度为0.5%的改性材料上甲苯、NO和N2O+NO2浓度最低, COx浓度最高.这表明Fe-0.5催化剂对甲苯和NO去除率最高, 对COx和N2选择性最好.与Mo改性实验结果类似, 当Fe浸渍液浓度提高后, 整体式催化剂的部分孔隙可能发生堵塞, 减小了催化反应接触面积, 从而导致活性和选择性降低.
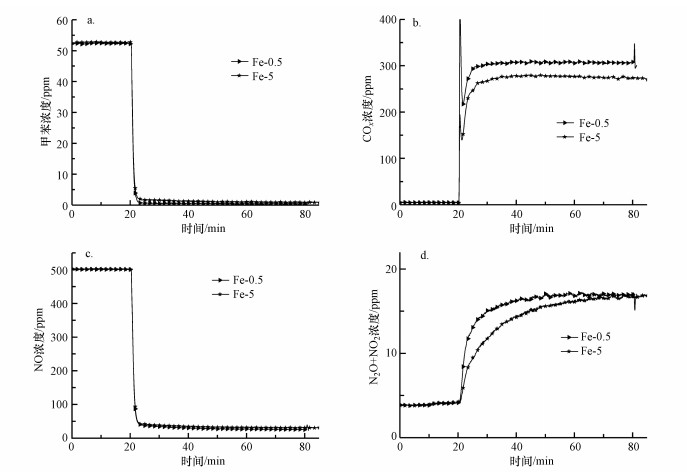 |
| 图 8 Fe浸渍液浓度对改性整体式催化剂在模拟烟气中对甲苯和NO同步去除的影响 (a.甲苯浓度, b.COx浓度, c.NO浓度, d.N2O+NO2浓度) Fig. 8 Effect of Fe impregnation solution concentration on simultaneous removal of toluene and NO in simulated flue gas over modified monolithic catalysts (a. concentration of toluene, b. concentration of COx, c. concentration of NO, d. concentration of N2O+NO2) |
采用单组分浸渍法制备了Cu浸渍液浓度分别为0.5%、5%的两种Cu改性整体式催化剂, 考察Cu浸渍液浓度对催化剂活性和选择性的影响.由图 9可知, 反应稳定60 min后, 两种改性整体式催化剂中, 浸渍液浓度为5%的整体式催化剂上甲苯、NO和N2O+NO2浓度最低, COx浓度最高.这表明Cu-5整体式催化剂对甲苯和NO去除率最高, 对N2和COx选择性最佳.该结论与Mo和Fe的结果不同, Cu改性材料在催化剂制备过程中, 材料内部发生的化学变化不同, 这可能是由于不同改性元素在煅烧过程中形成的晶粒尺寸不同及浸渍液在载体孔道中的扩散速率不同所导致.此外, 这还可以说明铜氧化物作为VOCs氧化的活性中心位点, 需要引入更多的量才能实现甲苯和NO的高效同步去除.
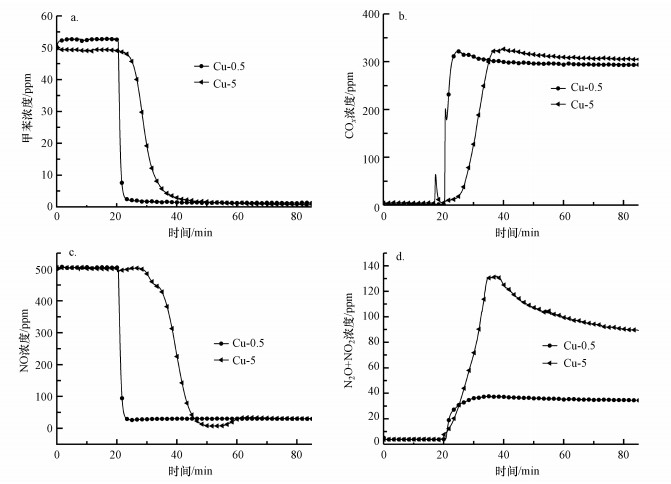 |
| 图 9 Cu浸渍液浓度对改性整体式催化剂在模拟烟气中对甲苯和NO同步去除的影响 (a.甲苯浓度, b.COx浓度, c.NO浓度, d.N2O+NO2浓度) Fig. 9 Effect of Cu impregnation solution concentration on simultaneous removal of toluene and NO in simulated flue gas over modified monolithic catalysts (a. concentration of toluene, b. concentration of COx, c. concentration of NO, d. concentration of N2O+NO2) |
根据前文研究确定采用单组分浸渍法制备烟气中甲苯和NO同步去除的改性催化材料.不同改性组分对整体式催化剂在模拟烟气中对甲苯和NO同步去除的影响如图 10所示.由图可知, 以甲苯为主的同步脱除反应中, 在NO去除率相似的前提下, Fe-0.5催化剂具有最优的活性和选择性, 其中, 甲苯去除率为99.3%, NO去除率为94.9%, COx和N2选择性分别为88.0%和96.4%.特别是与未改性的钒钨钛催化剂相比, COx选择性显著提高.结合烟气实际工况, 使用Fe-0.5改性钒钛基整体式催化剂可以实现甲苯和NO的高效同步去除.
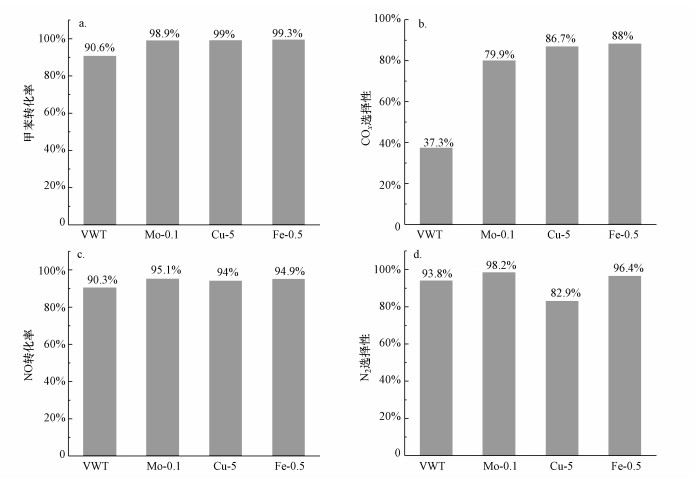 |
| 图 10 不同改性整体式催化剂对模拟烟气中甲苯和NO同步去除性能对比 (a.甲苯转化率, b.COx选择性, c.NO转化率, d.N2选择性) Fig. 10 Comparison of simultaneous removal performance of toluene and NO in simulated flue gas over different modified monolithic catalysts (a. conversion of toluene, b. selectivity of COx, c. conversion of NO, d. selectivity of N2) |
1) 采用工业上简便可行的单组分和多组分浸渍法制备了Mo/Cu/Fe改性钒钛基整体式催化剂, 研究了不同制备方法和浸渍液浓度对改性整体式催化剂在燃煤烟气中对甲苯和NO同步去除的性能.结果表明, 单组分浸渍法制备的浸渍液浓度为0.5%的Fe改性钒钛基催化剂对烟气中甲苯和NO同步去除性能最优, 在350 ℃下, 其甲苯转化率可达到99%, NO去除率为94.9%, COx和N2的选择性分别为88%和96.4%.
2) 表征结果表明, 改性组分均匀分散在钒钛基整体式催化剂表面, Fe改性的V2O5-WO3/TiO2催化剂对甲苯良好的氧化性能及对NO优异的还原能力归因于其具有最高的比表面积和孔容.
Chen L, Liao Y, Chen Y, et al. 2020. Performance of Ce-modified V-W-Ti type catalyst on simultaneous control of NO and typical VOCS[J]. Fuel Processing Technology, 207: 106483. DOI:10.1016/j.fuproc.2020.106483 |
Chen L, Liao Y, Xin S, et al. 2020. Simultaneous removal of NO and volatile organic compounds (VOCs) by Ce/Mo doping-modified selective catalytic reduction (SCR) catalysts in denitrification zone of coal-fired flue gas[J]. Fuel, 262: 116485. DOI:10.1016/j.fuel.2019.116485 |
Cheng J, Liu J, Wang T, et al. 2019. Reductions in volatile organic compound emissions from coal-fired power plants by combining air pollution control devices and modified fly ash[J]. Energy & Fuels, 33(4): 2926-2933. |
Hao R, Zhang Y, Wang Z, et al. 2017. An advanced wet method for simultaneous removal of SO2 and NO from coal-fired flue gas by utilizing a complex absorbent[J]. Chemical Engineering Journal, 307: 562-571. DOI:10.1016/j.cej.2016.08.103 |
He G, Zhang J, Hu Y, et al. 2019. Dual-template synthesis of mesoporous TiO2 nanotubes with structure-enhanced functional photocatalytic performance[J]. Applied Catalysis B: Environmental, 250: 301-312. DOI:10.1016/j.apcatb.2019.03.027 |
Huang X, Wang D, Yang Q, et al. 2021. Multi-pollutant control (MPC) of NO and chlorobenzene from industrial furnaces using a vanadia-based SCR catalyst[J]. Applied Catalysis B: Environmental, 285: 119835. DOI:10.1016/j.apcatb.2020.119835 |
Liu H, Fan Z, Sun C, et al. 2019. Improved activity and significant SO2 tolerance of samarium modified CeO2-TiO2 catalyst for NO selective catalytic reduction with NH3[J]. Applied Catalysis B: Environmental, 244: 671-683. DOI:10.1016/j.apcatb.2018.12.001 |
Liu J, Wang T, Cheng J, et al. 2019. Distribution of organic compounds in coal-fired power plant emissions[J]. Energy & Fuels, 33(6): 5430-5437. |
梁珑, 张玉冬, 文进军, 等. 2020. Pt-Cu/TiO2{001}纳米片用于CO2加氢制甲醇反应的研究[J]. 环境科学学报, 40(7): 2408-2416. |
Moreira Dos Santos C Y, de Almeida Azevedo D, de Aquino Neto F R. 2004. Atmospheric distribution of organic compounds from urban areas near a coal-fired power station[J]. Atmospheric Environment, 38(9): 1247-1257. DOI:10.1016/j.atmosenv.2003.11.026 |
Qin J, Wang J, Yang J, et al. 2020. Metal organic framework derivative-TiO2 composite as efficient and durable photocatalyst for the degradation of toluene[J]. Applied Catalysis B: Environmental, 267: 118667. DOI:10.1016/j.apcatb.2020.118667 |
Shao J, Lin F, Wang Z, et al. 2020. Low temperature catalytic ozonation of toluene in flue gas over Mn-based catalysts: Effect of support property and SO2/water vapor addition[J]. Applied Catalysis B: Environmental, 266: 118662. DOI:10.1016/j.apcatb.2020.118662 |
Shao J, Ma Q, Wang Z, et al. 2019. A superior liquid phase catalyst for enhanced absorption of NO2 together with SO2 after low temperature ozone oxidation for flue gas treatment[J]. Fuel, 247: 1-9. DOI:10.1016/j.fuel.2019.02.120 |
Shi J, Deng H, Bai Z, et al. 2015. Emission and profile characteristic of volatile organic compounds emitted from coke production, iron smelt, heating station and power plant in Liaoning Province, China[J]. Science of the Total Environment, 515-516: 101-108. DOI:10.1016/j.scitotenv.2015.02.034 |
Wang C, Li Y, Zhang C, et al. 2021. A simple strategy to improve Pd dispersion and enhance Pd/TiO2 catalytic activity for formaldehyde oxidation: The roles of surface defects[J]. Applied Catalysis B: Environmental, 282: 119540. DOI:10.1016/j.apcatb.2020.119540 |
Wang X, Yao Y, Gao W, et al. 2021. High-rate and high conductivity mesoporous TiO2 nano hollow spheres: Synergetic effect of structure and oxygen vacancies[J]. Ceramics International, 47(10): 13572-13581. DOI:10.1016/j.ceramint.2021.01.215 |
王玉亭, 石其其, 张铭洋, 等. 2020. 改性钒铈基催化剂催化氧化烟气中邻二甲苯[J]. 化工进展, 39(3): 1167-1173. |
Xu Y, Liu X, Wang H, et al. 2018. Influences of in-furnace kaolin addition on the formation and emission characteristics of PM2.5 in a 1000 MW coal-fired power station[J]. Environmental Science and Technology, 52(15): 8718-8724. DOI:10.1021/acs.est.8b02251 |
Yan Y, Yang C, Peng L, et al. 2016. Emission characteristics of volatile organic compounds from coal-, coal gangue-, and biomass-fired power plants in China[J]. Atmospheric Environment, 143: 261-269. DOI:10.1016/j.atmosenv.2016.08.052 |
Yang G, Zhao H, Luo X, et al. 2019. Promotion effect and mechanism of the addition of Mo on the enhanced low temperature SCR of NOx by NH3 over MnOx/γ-Al2O3 catalysts[J]. Applied Catalysis B: Environmental, 245: 743-752. DOI:10.1016/j.apcatb.2018.12.080 |
Ye Z, Giraudon J M, Nuns N, et al. 2018. Influence of the preparation method on the activity of copper-manganese oxides for toluene total oxidation[J]. Applied Catalysis B: Environmental, 223: 154-166. DOI:10.1016/j.apcatb.2017.06.072 |
Zeng Y, Wang Y, Meng Y, et al. 2019. The effect of preparation method on oxygen activation over Pt/TiO2 catalysts for toluene total oxidation[J]. Chemical Physics Letters, 730: 95-99. DOI:10.1016/j.cplett.2019.05.048 |
Zhang J, Hu Y, Qin J, et al. 2020. TiO2-UiO-66-NH2 nanocomposites as efficient photocatalysts for the oxidation of VOCs[J]. Chemical Engineering Journal, 385: 123814. DOI:10.1016/j.cej.2019.123814 |
Zhang Q, Song C, Lv G, et al. 2015. Effect of metal oxide partial substitution of V2O5 in V2O5-WO3/TiO2 on selective catalytic reduction of NO with NH3[J]. Journal of Industrial and Engineering Chemistry, 24: 79-86. DOI:10.1016/j.jiec.2014.09.012 |
 2021, Vol. 41
2021, Vol. 41


